COVID-19 Induced Acute Respiratory Distress Syndrome-A Multicenter Observational Study
- PMID: 33392222
- PMCID: PMC7775385
- DOI: 10.3389/fmed.2020.599533
COVID-19 Induced Acute Respiratory Distress Syndrome-A Multicenter Observational Study
Abstract
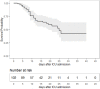
Background: Proportions of patients dying from the coronavirus disease-19 (COVID-19) vary between different countries. We report the characteristics; clinical course and outcome of patients requiring intensive care due to COVID-19 induced acute respiratory distress syndrome (ARDS). Methods: This is a retrospective, observational multicentre study in five German secondary or tertiary care hospitals. All patients consecutively admitted to the intensive care unit (ICU) in any of the participating hospitals between March 12 and May 4, 2020 with a COVID-19 induced ARDS were included. Results: A total of 106 ICU patients were treated for COVID-19 induced ARDS, whereas severe ARDS was present in the majority of cases. Survival of ICU treatment was 65.0%. Median duration of ICU treatment was 11 days; median duration of mechanical ventilation was 9 days. The majority of ICU treated patients (75.5%) did not receive any antiviral or anti-inflammatory therapies. Venovenous (vv) ECMO was utilized in 16.3%. ICU triage with population-level decision making was not necessary at any time. Univariate analysis associated older age, diabetes mellitus or a higher SOFA score on admission with non-survival during ICU stay. Conclusions: A high level of care adhering to standard ARDS treatments lead to a good outcome in critically ill COVID-19 patients.
Keywords: ARDS (acute respiratory distress syndrome); COVID-19; Germany; intensive care medicine; pandemia.
Copyright © 2020 Herrmann, Adam, Notz, Helmer, Sonntagbauer, Ungemach-Papenberg, Sanns, Zausig, Steinfeldt, Torje, Schmid, Schlesinger, Rolfes, Reyher, Kredel, Stumpner, Brack, Wurmb, Gill-Schuster, Kranke, Weismann, Klinker, Heuschmann, Rücker, Frantz, Ertl, Muellenbach, Mutlak, Meybohm, Zacharowski and Lotz.
Conflict of interest statement
PHeu reports grants from German Ministry of Research and Education, German Research Foundation, European Union, Charité—Universitätsmedizin Berlin, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert Koch Institute, German Heart Foundation, Federal Joint Committee (G-BA) within the Innovationfond, University Hospital Heidelberg (within RASUNOA-prime; supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo), Charité—Universitätsmedizin Berlin (within Mondafis; supported by an unrestricted research grant to the Charité from Bayer), University Göttingen (within FIND-AF randomized; supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), outside the submitted work. SF reports grants from DFG, BMBF, grants and personal fees from Abiomed, Amgen, Akzea, AstraZeneca, Bayer, Berlin-Chemie, Braun, Bristol-Myers Squibb, Boehringer, Daiichi Sankyo, MSD, Novartis, Pfizer, Sanofi-Aventis, Servier, Siemens, Zoll, outside the submitted work. GE reports grants and personal fees from Bayer, grants and personal fees from Novartis, grants and personal fees from Vifor Pharma Deutschland GmbH, outside the submitted work; PK reports other from FreseniusKabi, personal fees from BBraun, grants, personal fees and other from TEVARatiopharm, other from CSL Behring, other from Pajunk, other from APEPTICO Forschung und Entwicklung GmbH, outside the submitted work; KZ reports personal fees from Aesculap Akademie GmbH, personal fees from Affinites Sante, grants from Ashai Kasai Pharma, grants and personal fees from B. Braun AG, grants and personal fees from B. Braun Avitum AG, personal fees from Bayer AG, grants from Biotest AG, personal fees from Christian Doppler Stiftung, grants and personal fees from CSL Behring GmbH, personal fees from Cyto Sorbents GmbH, personal fees from Edward Lifescience Corporation, personal fees from Executive Insight AG, personal fees from Fresenius Kabi GmbH, personal fees from Fresenius Medical Care, personal fees from Haemonetics Corporation, personal fees from Hartmannbund Landesverband, personal fees from Health Adcances GmbH, personal fees from Heinen + Löwenstein GmbH, personal fees from Hexal AG, grants from INC Research, personal fees from Johnson and Johnson, personal fees from Josef Gassner, personal fees from Maquet GmbH, personal fees from Markus Lücke Kongress Organization, personal fees from Masimo International, personal fees from med Update GmbH, personal fees from Medizin and Markt Gesundheitswerk, personal fees from MSD Sharp and Dohme GmbH, personal fees from Nordic Group, personal fees from Nordic Pharma, grants from Novo Nordisc Pharma GmbH, grants from Pfizer Pharma GmbH, personal fees from Pharmacosmos, personal fees from Ratiopharm GmbH, personal fees from Salvia Medical GmbH, personal fees from Schering Stiftung, personal fees from Schöchl Medical Österreich, personal fees from Serumwerke, personal fees from Verlag für Printmedien und PR, Forum Sanitas, grants and personal fees from Vifor Pharma GmbH, personal fees from Wellington, personal fees from Werfen, outside the submitted work; HK served as a speaker and/or an Advisory Board Member for AbbVie, BMS, Gilead, Hexal, Janssen, MSD, Pfizer, ViiV and has received research funding from AbbVie, Arrowhaed, BMS, Gilead, Janssen, MSD, Novartis, German Liver Foundation, Hector Foundation, Virtual University of Bavaria, Federal Ministry of Education and Research, outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Available online at: https://coronavirus.jhu.edu/map.html (accessed on July 20, 2020).
-
- Available online at: https://www.sueddeutsche.de/bayern/coronavirus-bayern-erster-ausbruch-ru... (accessed on April 27, 2020).
-
- Available online at: https://coronavirus.jhu.edu/data/mortality (accessed on July 20, 2020).
LinkOut - more resources
Full Text Sources

