Multicenter registry of United States emergency department patients tested for SARS-CoV-2
- PMID: 33392542
- PMCID: PMC7771823
- DOI: 10.1002/emp2.12313
Multicenter registry of United States emergency department patients tested for SARS-CoV-2
Abstract
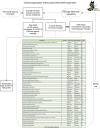
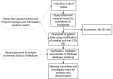
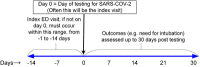
This paper summarizes the methodology for the registry of suspected COVID-19 in emergency care (RECOVER), a large clinical registry of patients from 155 United States (US) emergency departments (EDs) in 27 states tested for SARS-CoV-2 from March-September 2020. The initial goals are to derive and test: (1) a pretest probability instrument for prediction of SARS-CoV-2 test results, and from this instrument, a set of simple criteria to exclude COVID-19 (the COVID-19 Rule-Out Criteria-the CORC rule), and (2) a prognostic instrument for those with COVID-19. Patient eligibility included any ED patient tested for SARS-CoV-2 with a nasal or oropharyngeal swab. Abstracted clinical data included 204 variables representing the earliest manifestation of infection, including week of testing, demographics, symptoms, exposure risk, past medical history, test results, admission status, and outcomes 30 days later. In addition to the primary goals, the registry will provide a vital platform for characterizing the course, epidemiology, clinical features, and prognosis of patients tested for COVID-19 in the ED setting.
Keywords: COVID‐19; SARS‐CoV‐2; decision making; diagnosis; probability; prognosis; pulmonary embolism; registries; risk; venous thromboembolism; venous thrombosis.
© 2020 The Authors. JACEP Open published by Wiley Periodicals LLC on behalf of the American College of Emergency Physicians.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- U.S. Department of Health & Human Services CfDC. Coronavirus Disease 2019 (COVID‐19). https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Published 2020. Accessed October 8, 2020.
-
- Lipsitch M, Swerdlow DL, Finelli L. Defining the Epidemiology of Covid‐19 ‐ Studies Needed. N Engl J Med. 2020;382(13):1194–1196. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
