HSF-1 displays nuclear stress body formation in multiple tissues in Caenorhabditis elegans upon stress and following the transition to adulthood
- PMID: 33392968
- PMCID: PMC7925714
- DOI: 10.1007/s12192-020-01188-9
HSF-1 displays nuclear stress body formation in multiple tissues in Caenorhabditis elegans upon stress and following the transition to adulthood
Abstract
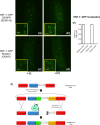
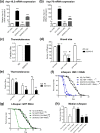
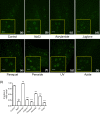
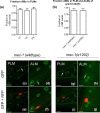
The transcription factor heat shock factor-1 (HSF-1) regulates the heat shock response (HSR), a cytoprotective response induced by proteotoxic stresses. Data from model organisms has shown that HSF-1 also has non-stress biological roles, including roles in the regulation of development and longevity. To better study HSF-1 function, we created a C. elegans strain containing HSF-1 tagged with GFP at its endogenous locus utilizing CRISPR/Cas9-guided transgenesis. We show that the HSF-1::GFP CRISPR worm strain behaves similarly to wildtype worms in response to heat and other stresses, and in other physiological processes. HSF-1 was expressed in all tissues assayed. Immediately following the initiation of reproduction, HSF-1 formed nuclear stress bodies, a hallmark of activation, throughout the germline. Upon the transition to adulthood, of HSF-1 nuclear stress bodies appeared in most somatic cells. Genetic loss of the germline suppressed nuclear stress body formation with age, suggesting that the germline influences HSF-1 activity. Interestingly, we found that various neurons did not form nuclear stress bodies after transitioning to adulthood. Therefore, the formation of HSF-1 nuclear stress bodies upon the transition to adulthood does not occur in a synchronous manner in all cell types. In sum, these studies enhance our knowledge of the expression and activity of the aging and proteostasis factor HSF-1 in a tissue-specific manner with age.
Keywords: Aging; C. elegans; Cell stress; HSF-1; Heat shock response; Nuclear stress bodies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

