BACH2-mediated FOS confers cytarabine resistance via stromal microenvironment alterations in pediatric ALL
- PMID: 33393145
- PMCID: PMC7935781
- DOI: 10.1111/cas.14792
BACH2-mediated FOS confers cytarabine resistance via stromal microenvironment alterations in pediatric ALL
Abstract
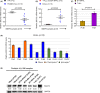
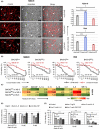
Acute lymphoblastic leukemia (ALL) is an aggressive hematological cancer that mainly affects children. Relapse and chemoresistance result in treatment failure, underlining the need for improved therapies. BTB and CNC homology 2 (BACH2) is a lymphoid-specific transcription repressor recognized as a tumor suppressor in lymphomas, but little is known about its function and regulatory network in pediatric ALL (p-ALL). Herein, we found aberrant BACH2 expression at new diagnosis not only facilitated risk stratification of p-ALL but also served as a sensitive predictor of early treatment response and clinical outcome. Silencing BACH2 in ALL cells increased cell proliferation and accelerated cell cycle progression. BACH2 blockade also promoted cell adhesion to bone marrow stromal cells and conferred cytarabine (Ara-C)-resistant properties to leukemia cells by altering stromal microenvironment. Strikingly, we identified FOS, a transcriptional activator competing with BACH2, as a novel downstream target repressed by BACH2. Blocking FOS by chemical compounds enhanced the effect of Ara-C treatment in both primary p-ALL cells and pre-B-ALL-driven leukemia xenografts and prolonged the survival of tumor-bearing mice. These data highlight an interconnected network of BACH2-FOS, disruption of which could render current chemotherapies more effective and offer a promising therapeutic strategy to overcome Ara-C resistance in p-ALL.
Keywords: BACH2; acute lymphoblastic leukemia; bone marrow microenvironment; childhood; cytarabine.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
BACH2-mediated CD28 and CD40LG axes contribute to pathogenesis and progression of T-cell lymphoblastic leukemia.Cell Death Dis. 2024 Jan 17;15(1):59. doi: 10.1038/s41419-024-06453-8. Cell Death Dis. 2024. PMID: 38233409 Free PMC article.
-
Ikaros regulation of the BCL6/BACH2 axis and its clinical relevance in acute lymphoblastic leukemia.Oncotarget. 2017 Jan 31;8(5):8022-8034. doi: 10.18632/oncotarget.14038. Oncotarget. 2017. PMID: 28030830 Free PMC article.
-
[Effects of bone marrow stromal cells on the chemotherapeutic sensitivity of acute lymphoblastic leukemia cells].Zhonghua Zhong Liu Za Zhi. 2017 Dec 23;39(12):885-890. doi: 10.3760/cma.j.issn.0253-3766.2017.12.002. Zhonghua Zhong Liu Za Zhi. 2017. PMID: 29262503 Chinese.
-
BTB domain and CNC homolog 2: A master regulator that controls immune response and cancer progression.Biochim Biophys Acta Rev Cancer. 2025 Jul;1880(3):189325. doi: 10.1016/j.bbcan.2025.189325. Epub 2025 Apr 17. Biochim Biophys Acta Rev Cancer. 2025. PMID: 40252853 Review.
-
BACH2-BCL6 balance regulates selection at the pre-B cell receptor checkpoint.Trends Immunol. 2014 Mar;35(3):131-7. doi: 10.1016/j.it.2013.11.002. Epub 2013 Dec 10. Trends Immunol. 2014. PMID: 24332591 Free PMC article. Review.
Cited by
-
3-Methyladenine but not antioxidants to overcome BACH2-mediated bortezomib resistance in mantle cell lymphoma.Cancer Cell Int. 2021 May 26;21(1):279. doi: 10.1186/s12935-021-01980-2. Cancer Cell Int. 2021. PMID: 34039348 Free PMC article.
-
BACH2-mediated CD28 and CD40LG axes contribute to pathogenesis and progression of T-cell lymphoblastic leukemia.Cell Death Dis. 2024 Jan 17;15(1):59. doi: 10.1038/s41419-024-06453-8. Cell Death Dis. 2024. PMID: 38233409 Free PMC article.
-
METTL3 mediates CPB1 expression by regulating transcription factor BACH2 to promote apoptosis and oxidative stress of lens epithelial cells.J Bioenerg Biomembr. 2025 Jun;57(2-3):161-171. doi: 10.1007/s10863-025-10054-1. Epub 2025 Feb 21. J Bioenerg Biomembr. 2025. PMID: 39982642
References
-
- Muto A, Tashiro S, Nakajima O, et al. The transcriptional programme of antibody class switching involves the repressor Bach2. Nature. 2004;429(6991):566‐571. - PubMed
-
- Itoh‐Nakadai A, Matsumoto M, Kato H, et al. A Bach2‐Cebp gene regulatory network for the commitment of multipotent hematopoietic progenitors. Cell Rep. 2017;18(10):2401‐2414. - PubMed
-
- Itoh‐Nakadai A, Hikota R, Muto A, et al. The transcription repressors Bach2 and Bach1 promote B cell development by repressing the myeloid program. Nat Immunol. 2014;15(12):1171‐1180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

