Metabolic requirements of pulmonary fibrosis: role of fibroblast metabolism
- PMID: 33393204
- PMCID: PMC8253875
- DOI: 10.1111/febs.15693
Metabolic requirements of pulmonary fibrosis: role of fibroblast metabolism
Abstract
Fibrosis is a pathologic condition characterized by excessive deposition of extracellular matrix and chronic scaring that can affect every organ system. Organ fibrosis is associated with significant morbidity and mortality, contributing to as many as 45% of all deaths in the developed world. In the lung, many chronic lung diseases may lead to fibrosis, the most devastating being idiopathic pulmonary fibrosis (IPF), which affects approximately 3 million people worldwide and has a median survival of 3.8 years. Currently approved therapies for IPF do not significantly extend lifespan, and thus, there is pressing need for novel therapeutic strategies to treat IPF and other fibrotic diseases. At the heart of pulmonary fibrosis are myofibroblasts, contractile cells with characteristics of both fibroblasts and smooth muscle cells, which are the primary cell type responsible for matrix deposition in fibrotic diseases. Much work has centered around targeting the extracellular growth factors and intracellular signaling regulators of myofibroblast differentiation. Recently, metabolic changes associated with myofibroblast differentiation have come to the fore as targetable mechanisms required for myofibroblast function. In this review, we will discuss the metabolic changes associated with myofibroblast differentiation, as well as the mechanisms by which these changes promote myofibroblast function. We will then discuss the potential for this new knowledge to lead to the development of novel therapies for IPF and other fibrotic diseases.
Keywords: bioenergetics; fibroblast; metabolism; mitochondria; pulmonary fibrosis.
© 2021 Federation of European Biochemical Societies.
Conflict of interest statement
Figures
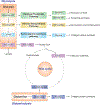
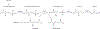

Similar articles
-
Featured Article: TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts.Exp Biol Med (Maywood). 2018 Apr;243(7):601-612. doi: 10.1177/1535370218761628. Epub 2018 Mar 4. Exp Biol Med (Maywood). 2018. PMID: 29504479 Free PMC article.
-
The Novel Small Molecule BTB Inhibits Pro-Fibrotic Fibroblast Behavior though Inhibition of RhoA Activity.Int J Mol Sci. 2022 Oct 8;23(19):11946. doi: 10.3390/ijms231911946. Int J Mol Sci. 2022. PMID: 36233248 Free PMC article.
-
Novel differences in gene expression and functional capabilities of myofibroblast populations in idiopathic pulmonary fibrosis.Am J Physiol Lung Cell Mol Physiol. 2018 Nov 1;315(5):L697-L710. doi: 10.1152/ajplung.00543.2017. Epub 2018 Aug 9. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 30091381
-
The role of metabolic reprogramming and de novo amino acid synthesis in collagen protein production by myofibroblasts: implications for organ fibrosis and cancer.Amino Acids. 2021 Dec;53(12):1851-1862. doi: 10.1007/s00726-021-02996-8. Epub 2021 May 8. Amino Acids. 2021. PMID: 33963932 Free PMC article. Review.
-
Idiopathic Pulmonary Fibrosis: An Update on Pathogenesis.Front Pharmacol. 2022 Jan 19;12:797292. doi: 10.3389/fphar.2021.797292. eCollection 2021. Front Pharmacol. 2022. PMID: 35126134 Free PMC article. Review.
Cited by
-
GLP-1R activation attenuates the progression of pulmonary fibrosis via disrupting NLRP3 inflammasome/PFKFB3-driven glycolysis interaction and histone lactylation.J Transl Med. 2024 Oct 21;22(1):954. doi: 10.1186/s12967-024-05753-z. J Transl Med. 2024. PMID: 39434134 Free PMC article.
-
Rational engineering of lung alveolar epithelium.NPJ Regen Med. 2023 Apr 28;8(1):22. doi: 10.1038/s41536-023-00295-2. NPJ Regen Med. 2023. PMID: 37117221 Free PMC article.
-
Targeting Growth Factor and Cytokine Pathways to Treat Idiopathic Pulmonary Fibrosis.Front Pharmacol. 2022 Jun 3;13:918771. doi: 10.3389/fphar.2022.918771. eCollection 2022. Front Pharmacol. 2022. PMID: 35721111 Free PMC article. Review.
-
Macrophage Implication in IPF: Updates on Immune, Epigenetic, and Metabolic Pathways.Cells. 2023 Sep 1;12(17):2193. doi: 10.3390/cells12172193. Cells. 2023. PMID: 37681924 Free PMC article. Review.
-
mTOR signaling regulates multiple metabolic pathways in human lung fibroblasts after TGF-β and in pulmonary fibrosis.Am J Physiol Lung Cell Mol Physiol. 2025 Feb 1;328(2):L215-L228. doi: 10.1152/ajplung.00189.2024. Epub 2025 Jan 2. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 39745695 Free PMC article.
References
-
- Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, Swigris JJ, Taniguchi H. & Wells AU (2017) Idiopathic pulmonary fibrosis, Nat Rev Dis Primers. 3, 17074. - PubMed
-
- Lederer DJ & Martinez FJ (2018) Idiopathic Pulmonary Fibrosis, N Engl J Med. 378, 1811–1823. - PubMed
-
- Richeldi L, Collard HR & Jones MG (2017) Idiopathic pulmonary fibrosis, Lancet. 389, 1941–1952. - PubMed
-
- Gabbiani G, Ryan GB & Majne G. (1971) Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction, Experientia. 27, 549–50. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

