Insulin signaling in health and disease
- PMID: 33393497
- PMCID: PMC7773347
- DOI: 10.1172/JCI142241
Insulin signaling in health and disease
Abstract
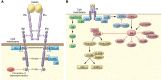
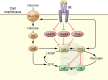
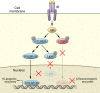
The molecular mechanisms of cellular insulin action have been the focus of much investigation since the discovery of the hormone 100 years ago. Insulin action is impaired in metabolic syndrome, a condition known as insulin resistance. The actions of the hormone are initiated by binding to its receptor on the surface of target cells. The receptor is an α2β2 heterodimer that binds to insulin with high affinity, resulting in the activation of its tyrosine kinase activity. Once activated, the receptor can phosphorylate a number of intracellular substrates that initiate discrete signaling pathways. The tyrosine phosphorylation of some substrates activates phosphatidylinositol-3-kinase (PI3K), which produces polyphosphoinositides that interact with protein kinases, leading to activation of the kinase Akt. Phosphorylation of Shc leads to activation of the Ras/MAP kinase pathway. Phosphorylation of SH2B2 and of Cbl initiates activation of G proteins such as TC10. Activation of Akt and other protein kinases produces phosphorylation of a variety of substrates, including transcription factors, GTPase-activating proteins, and other kinases that control key metabolic events. Among the cellular processes controlled by insulin are vesicle trafficking, activities of metabolic enzymes, transcriptional factors, and degradation of insulin itself. Together these complex processes are coordinated to ensure glucose homeostasis.
Conflict of interest statement
Figures
References
-
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

