Keratin 19 maintains E-cadherin localization at the cell surface and stabilizes cell-cell adhesion of MCF7 cells
- PMID: 33393839
- PMCID: PMC7801129
- DOI: 10.1080/19336918.2020.1868694
Keratin 19 maintains E-cadherin localization at the cell surface and stabilizes cell-cell adhesion of MCF7 cells
Abstract
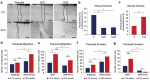
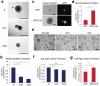
A cytoskeletal protein keratin 19 (K19) is highly expressed in breast cancer but its effects on breast cancer cell mechanics are unclear. In MCF7 cells where K19 expression is ablated,we found that K19 is required to maintain rounded epithelial-like shape and tight cell-cell adhesion. A loss of K19 also lowered cell surface E-cadherin levels. Inhibiting internalization restored cell-cell adhesion of KRT19 knockout cells, suggesting that E-cadherin internalization contributed to defective adhesion. Ultimately, while K19 inhibited cell migration and invasion, it was required for cells to form colonies in suspension. Our results suggest that K19 stabilizes E-cadherin complexes at the cell membrane to maintain cell-cell adhesion which inhibits cell invasiveness but provides growth and survival advantages for circulating tumor cells.
Keywords: Intermediate filaments; adherens junction; breast cancer; cell migration; cell morphology; cell-cell adhesion; keratin; keratin 19; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
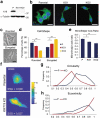

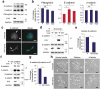
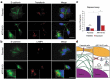
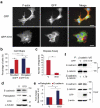
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
