Investigating the Mechanism of Trimethoprim-Induced Skin Rash and Liver Injury
- PMID: 33394045
- PMCID: PMC7916736
- DOI: 10.1093/toxsci/kfaa182
Investigating the Mechanism of Trimethoprim-Induced Skin Rash and Liver Injury
Abstract
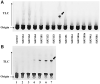
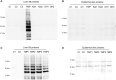
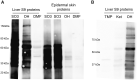
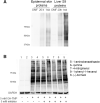
Trimethoprim (TMP)-induced skin rash and liver injury are likely to involve the formation of reactive metabolites. Analogous to nevirapine-induced skin rash, 1 possible reactive metabolite is the sulfate conjugate of α-hydroxyTMP, a metabolite of TMP. We synthesized this sulfate and found that it reacts with proteins in vitro. We produced a TMP-antiserum and found covalent binding of TMP in the liver of TMP-treated rats. However, we found that α-hydroxyTMP is not a substrate for human sulfotransferases, and we did not detect covalent binding in the skin of TMP-treated rats. Although less reactive than the sulfate, α-hydroxyTMP was found to covalently bind to liver and skin proteins in vitro. Even though there was covalent binding to liver proteins, TMP did not cause liver injury in rats or in our impaired immune tolerance mouse model that has been able to unmask the ability of other drugs to cause immune-mediated liver injury. This is likely because there was much less covalent binding of TMP in the livers of TMP-treated mice than TMP-treated rats. It is possible that some patients have a sulfotransferase that can produce the reactive benzylic sulfate; however, α-hydroxyTMP, itself, has sufficient reactivity to covalently bind to proteins in the skin and may be responsible for TMP-induced skin rash. Interspecies and interindividual differences in TMP metabolism may be 1 factor that determines the risk of TMP-induced skin rash. This study provides important data required to understand the mechanism of TMP-induced skin rash and drug-induced skin rash in general.
Keywords: covalent binding; idiosyncratic drug reaction; reactive metabolite; skin rash; sulfotransferase.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society of Toxicology.
Figures



References
-
- Baker C. A., Uno H., Johnson G. A. (1994). Minoxidil sulfation in the hair follicle. Skin Pharmacol. Physiol. 7, 335–339. - PubMed
-
- Brogden R. N., Carmine A. A., Heel R. C., Speight T. M., Avery G. S. (1982). Trimethoprim: A review of its antibacterial activity, pharmacokinetics and therapeutic use in urinary tract infections. Drugs 23, 405–430. - PubMed
-
- Fuchs E. (1983). Evolution and complexity of the genes encoding the keratins of human epidermal cells. J. Invest. Dermatol. 81, S141–S144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

