Negative Fusional Vergence Is Abnormal in Children with Symptomatic Convergence Insufficiency
- PMID: 33394929
- PMCID: PMC7789288
- DOI: 10.1097/OPX.0000000000001626
Negative Fusional Vergence Is Abnormal in Children with Symptomatic Convergence Insufficiency
Abstract
Significance: Deficits of disparity divergence found with objective eye movement recordings may not be apparent with standard clinical measures of negative fusional vergence (NFV) in children with symptomatic convergence insufficiency.
Purpose: This study aimed to determine whether NFV is normal in untreated children with symptomatic convergence insufficiency and whether NFV improves after vergence/accommodative therapy.
Methods: This secondary analysis of NFV measures before and after office-based vergence/accommodative therapy reports changes in (1) objective eye movement recording responses to 4° disparity divergence step stimuli from 12 children with symptomatic convergence insufficiency compared with 10 children with normal binocular vision (NBV) and (2) clinical NFV measures in 580 children successfully treated in three Convergence Insufficiency Treatment Trial studies.
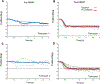
Results: At baseline, the Convergence Insufficiency Treatment Trial cohort's mean NFV break (14.6 ± 4.8Δ) and recovery (10.6 ± 4.2Δ) values were significantly greater (P < .001) than normative values. The post-therapy mean improvements for blur, break, and recovery of 5.2, 7.2, and 1.3Δ, respectively, were statistically significant (P < .0001). Mean pre-therapy responses to 4° disparity divergence step stimuli were worse in the convergence insufficiency group compared with the NBV group for peak velocity (P < .001), time to peak velocity (P = .01), and response amplitude (P < .001). After therapy, the convergence insufficiency group showed statistically significant improvements in mean peak velocity (11.63°/s; 95% confidence interval [CI], 6.6 to 16.62°/s), time to peak velocity (-0.12 seconds; 95% CI, -0.19 to -0.05 seconds), and response amplitude (1.47°; 95% CI, 0.83 to 2.11°), with measures no longer statistically different from the NBV cohort (P > .05).
Conclusions: Despite clinical NFV measurements that seem greater than normal, children with symptomatic convergence insufficiency may have deficient NFV when measured with objective eye movement recordings. Both objective and clinical measures of NFV can be improved with vergence/accommodative therapy.
Copyright © 2020 American Academy of Optometry.
Conflict of interest statement
Conflict of Interest Disclosure: None of the authors have reported a financial conflict of interest.
Figures




References
-
- von Noorden G Burian-Von Noorden’s Binocular Vision and Ocular Motility: Theory and Management of Strabismus, 6th ed. St. Louis: C.V. Mosby; 2001.
-
- Griffin JR, Grisham JD, Borsting EJ. Binocular Anomalies: Theory, Testing and Therapy, 4th ed. Santa Ana, CA: Optometric Extension Program Foundation; 2010.
-
- Scheiman M, Wick B. Clinical Management of Binocular Vision: Heterophoric, Accommodative and Eye Movement Disorders, 5th ed. Philadelphia: Wolters-Kluwer; 2019.
-
- Scheiman M, Mitchell GL, Cotter S, et al. A Randomized Trial of the Effectiveness of Treatments for Convergence Insufficiency in Children. Arch Ophthalmol 2005;123:14–24. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

