Neuron-Glia Interaction in the Developing and Adult Enteric Nervous System
- PMID: 33396231
- PMCID: PMC7823798
- DOI: 10.3390/cells10010047
Neuron-Glia Interaction in the Developing and Adult Enteric Nervous System
Abstract
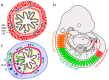
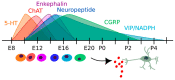
The enteric nervous system (ENS) constitutes the largest part of the peripheral nervous system. In recent years, ENS development and its neurogenetic capacity in homeostasis and allostasishave gained increasing attention. Developmentally, the neural precursors of the ENS are mainly derived from vagal and sacral neural crest cell portions. Furthermore, Schwann cell precursors, as well as endodermal pancreatic progenitors, participate in ENS formation. Neural precursorsenherite three subpopulations: a bipotent neuron-glia, a neuronal-fated and a glial-fated subpopulation. Typically, enteric neural precursors migrate along the entire bowel to the anal end, chemoattracted by glial cell-derived neurotrophic factor (GDNF) and endothelin 3 (EDN3) molecules. During migration, a fraction undergoes differentiation into neurons and glial cells. Differentiation is regulated by bone morphogenetic proteins (BMP), Hedgehog and Notch signalling. The fully formed adult ENS may react to injury and damage with neurogenesis and gliogenesis. Nevertheless, the origin of differentiating cells is currently under debate. Putative candidates are an embryonic-like enteric neural progenitor population, Schwann cell precursors and transdifferentiating glial cells. These cells can be isolated and propagated in culture as adult ENS progenitors and may be used for cell transplantation therapies for treating enteric aganglionosis in Chagas and Hirschsprung's diseases.
Keywords: BMP; EDN3; GDNF; Notch; enteric nervous system; gastrointestinal system; gut; hedgehog; neural crest cells; neuron-glia interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Furness J.B. Enteric Nervous System. Scholarpedia. 2007;2:4064. doi: 10.4249/scholarpedia.4064. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

