Structural Diversity of Peptoids: Tube-Like Structures of Macrocycles
- PMID: 33396246
- PMCID: PMC7795174
- DOI: 10.3390/molecules26010150
Structural Diversity of Peptoids: Tube-Like Structures of Macrocycles
Abstract
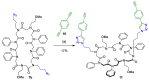
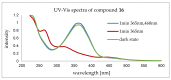
Peptoids, or poly-N-substituted glycines, are characterised by broad structural diversity. Compared to peptides, they are less restricted in rotation and lack backbone-derived H bonding. Nevertheless, certain side chains force the peptoid backbone into distinct conformations. Designable secondary structures like helices or nanosheets arise from this knowledge. Herein, we report the copper-catalysed alkyne-azide cycloaddition (CuAAC) of macrocycles to form innovative tube-like tricyclic peptoids, giving access to host-guest chemistry or storage applications. Different linker systems make the single tubes tuneable in size and enable modifications within the gap. An azobenzene linker, which is reversibly switchable in conformation, was successfully incorporated and allowed for light-triggered changes of the entire tricyclic structure.
Keywords: CuAAC; foldamers; peptidomimetics; tricyclic peptoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


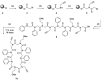







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

