Modulatory Effects of Osthole on Lipopolysaccharides-Induced Inflammation in Caco-2 Cell Monolayer and Co-Cultures with THP-1 and THP-1-Derived Macrophages
- PMID: 33396265
- PMCID: PMC7824174
- DOI: 10.3390/nu13010123
Modulatory Effects of Osthole on Lipopolysaccharides-Induced Inflammation in Caco-2 Cell Monolayer and Co-Cultures with THP-1 and THP-1-Derived Macrophages
Abstract
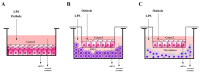
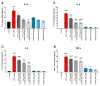
Lipopolysaccharydes (LPS) are responsible for the intestinal inflammatory reaction, as they may disrupt tight junctions and induce cytokines (CKs) secretion. Osthole has a wide spectrum of pharmacological effects, thus its anti-inflammatory potential in the LPS-treated Caco-2 cell line as well as in Caco-2/THP-1 and Caco-2/macrophages co-cultures was investigated. In brief, Caco-2 cells and co-cultures were incubated with LPS to induce an inflammatory reaction, after which osthole (150-450 ng/mL) was applied to reduce this effect. After 24 h, the level of secreted CKs and changes in gene expression were examined. LPS significantly increased the levels of IL-1β, -6, -8, and TNF-α, while osthole reduced this effect in a concentration-dependent manner, with the most significant decrease when a 450 ng/mL dose was applied (p < 0.0001). A similar trend was observed in changes in gene expression, with the significant osthole efficiency at a concentration of 450 ng/μL for IL1R1 and COX-2 (p < 0.01) and 300 ng/μL for NF-κB (p < 0.001). Osthole increased Caco-2 monolayer permeability, thus if it would ever be considered as a potential drug for minimizing intestinal inflammatory symptoms, its safety should be confirmed in extended in vitro and in vivo studies.
Keywords: gene expression; interleukin; permeability; pro-inflammatory cytokine; transepithelial electrical resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Cocetta V., Catanzaro D., Borgonetti V., Ragazzi E., Giron M.C., Governa P., Carnevali I., Biagi M., Montopoli M. A Fixed Combination of Probiotics and Herbal Extracts Attenuates Intestinal Barrier Dysfunction from Inflammatory Stress in an In vitro Model Using Caco-2 Cells. Recent Pat. Food Nutr. Agric. 2019;10:62–69. doi: 10.2174/2212798410666180808121328. - DOI - PubMed
-
- Governa P., Marchi M., Cocetta V., De Leo B., Saunders P.T.K., Catanzaro D., Miraldi E., Montopoli M., Biagi M. Effects of Boswellia Serrata Roxb. and Curcuma longa L. in an In Vitro Intestinal Inflammation Model Using Immune Cells and Caco-2. Pharmaceuticals. 2018;11:126. doi: 10.3390/ph11040126. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

