Optimizations of In Vitro Mucus and Cell Culture Models to Better Predict In Vivo Gene Transfer in Pathological Lung Respiratory Airways: Cystic Fibrosis as an Example
- PMID: 33396283
- PMCID: PMC7823756
- DOI: 10.3390/pharmaceutics13010047
Optimizations of In Vitro Mucus and Cell Culture Models to Better Predict In Vivo Gene Transfer in Pathological Lung Respiratory Airways: Cystic Fibrosis as an Example
Abstract
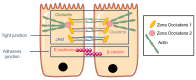
The respiratory epithelium can be affected by many diseases that could be treated using aerosol gene therapy. Among these, cystic fibrosis (CF) is a lethal inherited disease characterized by airways complications, which determine the life expectancy and the effectiveness of aerosolized treatments. Beside evaluations performed under in vivo settings, cell culture models mimicking in vivo pathophysiological conditions can provide complementary insights into the potential of gene transfer strategies. Such models must consider multiple parameters, following the rationale that proper gene transfer evaluations depend on whether they are performed under experimental conditions close to pathophysiological settings. In addition, the mucus layer, which covers the epithelial cells, constitutes a physical barrier for gene delivery, especially in diseases such as CF. Artificial mucus models featuring physical and biological properties similar to CF mucus allow determining the ability of gene transfer systems to effectively reach the underlying epithelium. In this review, we describe mucus and cellular models relevant for CF aerosol gene therapy, with a particular emphasis on mucus rheology. We strongly believe that combining multiple pathophysiological features in single complex cell culture models could help bridge the gaps between in vitro and in vivo settings, as well as viral and non-viral gene delivery strategies.
Keywords: airway epithelium; cystic fibrosis; gene delivery; in vitro model; mucus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ratjen F., Rietschel E., Griese M., Ballmann M., Kleinau I., Doring G., Reinhardt D., Paul K. Fractional analysis of bronchoalveolar lavage fluid cytology in cystic fibrosis patients with normal lung function. Bronchoalveolar lavage for the evaluation of anti-inflammatory treatment (BEAT) study group. Eur. Respir. J. 2000;15:141–145. doi: 10.1183/09031936.00.15114100. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

