Molecular Mechanisms of Treatment Resistance in Glioblastoma
- PMID: 33396284
- PMCID: PMC7794986
- DOI: 10.3390/ijms22010351
Molecular Mechanisms of Treatment Resistance in Glioblastoma
Abstract
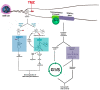
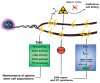
Glioblastoma is the most common malignant primary brain tumor in adults and is almost invariably fatal. Despite our growing understanding of the various mechanisms underlying treatment failure, the standard-of-care therapy has not changed over the last two decades, signifying a great unmet need. The challenges of treating glioblastoma are many and include inadequate drug or agent delivery across the blood-brain barrier, abundant intra- and intertumoral heterogeneity, redundant signaling pathways, and an immunosuppressive microenvironment. Here, we review the innate and adaptive molecular mechanisms underlying glioblastoma's treatment resistance, emphasizing the intrinsic challenges therapeutic interventions must overcome-namely, the blood-brain barrier, tumoral heterogeneity, and microenvironment-and the mechanisms of resistance to conventional treatments, targeted therapy, and immunotherapy.
Keywords: chemoresistance; glioblastoma; heterogeneity; immunotherapy; radioresistance; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Westphal M., Hilt D.C., Bortey E., Delavault P., Olivares R., Warnke P.C., Whittle I.R., Jääskeläinen J., Ram Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1093/neuonc/5.2.79. - DOI - PMC - PubMed
-
- Kreisl T.N., Kim L., Moore K., Duic P., Royce C., Stroud I., Garren N., Mackey M., Butman J.A., Camphausen K., et al. Phase II Trial of Single-Agent Bevacizumab Followed by Bevacizumab Plus Irinotecan at Tumor Progression in Recurrent Glioblastoma. J. Clin. Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. - DOI - PMC - PubMed
-
- Stupp R., Taillibert S., Kanner A.A., Kesari S., Steinberg D.M., Toms S.A., Taylor L.P., Lieberman F., Silvani A., Fink K.L., et al. Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastomaa: A Randomized Clinical Trial. JAMA. 2015;314:2535–2543. doi: 10.1001/jama.2015.16669. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

