Lead SARS-CoV-2 Candidate Vaccines: Expectations from Phase III Trials and Recommendations Post-Vaccine Approval
- PMID: 33396343
- PMCID: PMC7824305
- DOI: 10.3390/v13010054
Lead SARS-CoV-2 Candidate Vaccines: Expectations from Phase III Trials and Recommendations Post-Vaccine Approval
Abstract
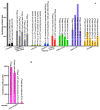
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is transmitted primarily through respiratory droplets/aerosols and it causes COVID-19. The virus infects epithelial cells by using the spike protein on its surface to bind to angiotensin-converting enzyme 2 receptor on the cells. Thus, candidate vaccines targeting the spike protein are currently being developed to prevent against infections. Approximately 44 SARS-CoV-2 candidate vaccines are in clinical trials (phase I-III) and an additional 164 candidates are in preclinical stages. The efficacy data from phase I/II trials of lead candidate vaccines look very promising with virus-neutralizing geometric mean antibody titers in the range of 16.6-3906. Most recently, two SARS-CoV-2 candidate vaccines, BNT162b2 and mRNA-1273, have been granted the first emergency use authorization (EUA) in the U.S.; BNT162b2 has also been granted an EUA in the United Kingdom, Canada, and in the European Union. This review assesses whether SARS-CoV-2 candidate vaccines (with approved EUA or in phase III trials) meet the criteria for an ideal SARS-CoV-2 vaccine. The review concludes with expectations from phase III trials and recommendations for phase IV studies (post-vaccine approval).
Keywords: COVID-19; SARS-CoV-2; SARS-CoV-2 vaccines; clinical trials.
Conflict of interest statement
Ebenezer Tumban is a co-inventor of an L2-bacteriophage VLP-related patent application licensed to Agilvax Biotech. Interactions with Agilvax are managed by the University of New Mexico in accordance with its conflict of interest policies.
Figures
References
-
- Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., McHugh T.D., Memish Z.A., Drosten C., et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. - DOI - PMC - PubMed
-
- JHU COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) [(accessed on 1 December 2020)]; Available online: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd4029942....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

