Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair
- PMID: 33396359
- PMCID: PMC7824389
- DOI: 10.3390/cells10010051
Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair
Abstract
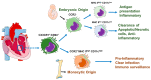
The immune system plays a pivotal role in the initiation, development and resolution of inflammation following insult or damage to organs. The heart is a vital organ which supplies nutrients and oxygen to all parts of the body. Heart failure (HF) has been conventionally described as a disease associated with cardiac tissue damage caused by systemic inflammation, arrhythmia and conduction defects. Cardiac inflammation and subsequent tissue damage is orchestrated by the infiltration and activation of various immune cells including neutrophils, monocytes, macrophages, eosinophils, mast cells, natural killer cells, and T and B cells into the myocardium. After tissue injury, monocytes and tissue-resident macrophages undergo marked phenotypic and functional changes, and function as key regulators of tissue repair, regeneration and fibrosis. Disturbance in resident macrophage functions such as uncontrolled production of inflammatory cytokines, growth factors and inefficient generation of an anti-inflammatory response or unsuccessful communication between macrophages and epithelial and endothelial cells and fibroblasts can lead to aberrant repair, persistent injury, and HF. Therefore, in this review, we discuss the role of cardiac macrophages on cardiac inflammation, tissue repair, regeneration and fibrosis.
Keywords: cardiac inflammation; cardiac macrophages; fibrosis; tissue repair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

