Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases
- PMID: 33396382
- PMCID: PMC7795229
- DOI: 10.3390/ijms22010362
Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases
Abstract
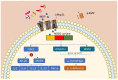
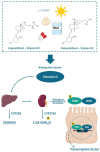
Inflammatory bowel disease (IBD) is a chronic inflammation of the gastrointestinal tract (GIT), including Crohn's disease (CD) and ulcerative colitis (UC), which differ in the location and lesion extensions. Both diseases are associated with microbiota dysbiosis, with a reduced population of butyrate-producing species, abnormal inflammatory response, and micronutrient deficiency (e.g., vitamin D hypovitaminosis). Vitamin D (VitD) is involved in immune cell differentiation, gut microbiota modulation, gene transcription, and barrier integrity. Vitamin D receptor (VDR) regulates the biological actions of the active VitD (1α,25-dihydroxyvitamin D3), and is involved in the genetic, environmental, immune, and microbial aspects of IBD. VitD deficiency is correlated with disease activity and its administration targeting a concentration of 30 ng/mL may have the potential to reduce disease activity. Moreover, VDR regulates functions of T cells and Paneth cells and modulates release of antimicrobial peptides in gut microbiota-host interactions. Meanwhile, beneficial microbial metabolites, e.g., butyrate, upregulate the VDR signaling. In this review, we summarize the clinical progress and mechanism studies on VitD/VDR related to gut microbiota modulation in IBD. We also discuss epigenetics in IBD and the probiotic regulation of VDR. Furthermore, we discuss the existing challenges and future directions. There is a lack of well-designed clinical trials exploring the appropriate dose and the influence of gender, age, ethnicity, genetics, microbiome, and metabolic disorders in IBD subtypes. To move forward, we need well-designed therapeutic studies to examine whether enhanced vitamin D will restore functions of VDR and microbiome in inhibiting chronic inflammation.
Keywords: Crohn’s disease; VDR; antimicrobial peptides (AMP); dysbiosis; epigenetics; inflammation; metabolites; microbiome; micronutrient; nuclear receptor; probiotics; tight junctions; ulcerative colitis; vitamin D.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- AGA Patient Information Section Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2017;15:A21. doi: 10.1016/S1542-3565(17)30640-7. - DOI
-
- Alatab S., Sepanlou S.G., Ikuta K., Vahedi H., Bisignano C., Safiri S., Sadeghi A., Nixon M.R., Abdoli A., Abolhassani H., et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

