Polycyclic Aromatic Hydrocarbons Activate the Aryl Hydrocarbon Receptor and the Constitutive Androstane Receptor to Regulate Xenobiotic Metabolism in Human Liver Cells
- PMID: 33396476
- PMCID: PMC7796163
- DOI: 10.3390/ijms22010372
Polycyclic Aromatic Hydrocarbons Activate the Aryl Hydrocarbon Receptor and the Constitutive Androstane Receptor to Regulate Xenobiotic Metabolism in Human Liver Cells
Abstract
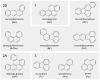
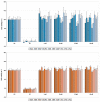
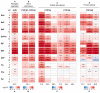
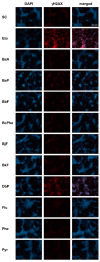
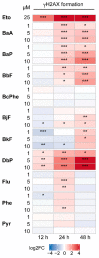
Polycyclic aromatic hydrocarbons (PAHs) are environmental pollutants produced by incomplete combustion of organic matter. They induce their own metabolism by upregulating xenobiotic-metabolizing enzymes such as cytochrome P450 monooxygenase 1A1 (CYP1A1) by activating the aryl hydrocarbon receptor (AHR). However, previous studies showed that individual PAHs may also interact with the constitutive androstane receptor (CAR). Here, we studied ten PAHs, different in carcinogenicity classification, for their potential to activate AHR- and CAR-dependent luciferase reporter genes in human liver cells. The majority of investigated PAHs activated AHR, while non-carcinogenic PAHs tended to activate CAR. We further characterized gene expression, protein abundancies and activities of the AHR targets CYP1A1 and 1A2, and the CAR target CYP2B6 in human HepaRG hepatoma cells. Enzyme induction patterns strongly resembled the profiles obtained at the receptor level, with AHR-activating PAHs inducing CYP1A1/1A2 and CAR-activating PAHs inducing CYP2B6. In summary, this study provides evidence that beside well-known activation of AHR, some PAHs also activate CAR, followed by subsequent expression of respective target genes. Furthermore, we found that an increased PAH ring number is associated with AHR activation as well as the induction of DNA double-strand breaks, whereas smaller PAHs activated CAR but showed no DNA-damaging potential.
Keywords: liver; nuclear receptors; polycyclic aromatic hydrocarbons; toxicity; xenobiotic metabolism.
Conflict of interest statement
The authors declare the following competing financial interest: Oliver Poetz is a shareholder of SIGNATOPE GmbH. SIGNATOPE offers assay development and service using MS-based immunoassay technology.
Figures

References
-
- Boström C.E., Gerde P., Hanberg A., Jernstrom B., Johansson C., Kyrklund T., Rannug A., Tornqvist M., Victorin K., Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 2002;110(Suppl. S3):451–488. doi: 10.1289/ehp.110-1241197. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

