Convection Enhanced Delivery of Topotecan for Gliomas: A Single-Center Experience
- PMID: 33396668
- PMCID: PMC7823846
- DOI: 10.3390/pharmaceutics13010039
Convection Enhanced Delivery of Topotecan for Gliomas: A Single-Center Experience
Abstract
A key limitation to glioma treatment involves the blood brain barrier (BBB). Convection enhanced delivery (CED) is a technique that uses a catheter placed directly into the brain parenchyma to infuse treatments using a pressure gradient. In this manuscript, we describe the physical principles behind CED along with the common pitfalls and methods for optimizing convection. Finally, we highlight our institutional experience using topotecan CED for the treatment of malignant glioma.
Keywords: convection enhanced delivery; glioblastoma; glioma; topotecan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


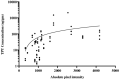
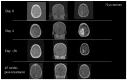

References
-
- Gill B.J., Pisapia D.J., Malone H.R., Goldstein H., Lei L., Sonabend A., Yun J., Samanamud J., Sims J.S., Banu M., et al. MRI-localized biopsies reveal subtype-specific differences in molecular and cellular composition at the margins of glioblastoma. Proc. Natl. Acad. Sci. USA. 2014;111:12550–12555. doi: 10.1073/pnas.1405839111. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

