Mast Cell Chymase and Kidney Disease
- PMID: 33396702
- PMCID: PMC7795820
- DOI: 10.3390/ijms22010302
Mast Cell Chymase and Kidney Disease
Abstract
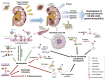
A sizable part (~2%) of the human genome encodes for proteases. They are involved in many physiological processes, such as development, reproduction and inflammation, but also play a role in pathology. Mast cells (MC) contain a variety of MC specific proteases, the expression of which may differ between various MC subtypes. Amongst these proteases, chymase represents up to 25% of the total proteins in the MC and is released from cytoplasmic granules upon activation. Once secreted, it cleaves the targets in the local tissue environment, but may also act in lymph nodes infiltrated by MC, or systemically, when reaching the circulation during an inflammatory response. MC have been recognized as important components in the development of kidney disease. Based on this observation, MC chymase has gained interest following the discovery that it contributes to the angiotensin-converting enzyme's independent generation of angiotensin II, an important inflammatory mediator in the development of kidney disease. Hence, progress regarding its role has been made based on studies using inhibitors but also on mice deficient in MC protease 4 (mMCP-4), the functional murine counterpart of human chymase. In this review, we discuss the role and actions of chymase in kidney disease. While initially believed to contribute to pathogenesis, the accumulated data favor a more subtle view, indicating that chymase may also have beneficial actions.
Keywords: angiotensin II; inflammation; kidney disease; mast cell; mast cell chymase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

