Monounsaturated Fatty Acids in Obesity-Related Inflammation
- PMID: 33396940
- PMCID: PMC7795523
- DOI: 10.3390/ijms22010330
Monounsaturated Fatty Acids in Obesity-Related Inflammation
Abstract
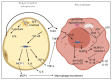
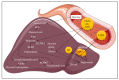
Obesity is an important aspect of the metabolic syndrome and is often associated with chronic inflammation. In this context, inflammation of organs participating in energy homeostasis (such as liver, adipose tissue, muscle and pancreas) leads to the recruitment and activation of macrophages, which secrete pro-inflammatory cytokines. Interleukin-1β secretion, sustained C-reactive protein plasma levels and activation of the NLRP3 inflammasome characterize this inflammation. The Stearoyl-CoA desaturase-1 (SCD1) enzyme is a central regulator of lipid metabolism and fat storage. This enzyme catalyzes the generation of monounsaturated fatty acids (MUFAs)-major components of triglycerides stored in lipid droplets-from saturated fatty acid (SFA) substrates. In this review, we describe the molecular effects of specific classes of fatty acids (saturated and unsaturated) to better understand the impact of different diets (Western versus Mediterranean) on inflammation in a metabolic context. Given the beneficial effects of a MUFA-rich Mediterranean diet, we also present the most recent data on the role of SCD1 activity in the modulation of SFA-induced chronic inflammation.
Keywords: chronic inflammation; metabolic syndrome; monounsaturated fatty acids (MUFA); saturated fatty acid (SFA); stearoyl-CoA desaturase-1 (SCD1).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Goodpaster B.H., Krishnaswami S., Harris T.B., Katsiaras A., Kritchevsky S.B., Simonsick E.M., Nevitt M., Holvoet P., Newman A.B. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch. Intern. Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

