Dose-Escalation Study of Systemically Delivered rAAVrh74.MHCK7.micro-dystrophin in the mdx Mouse Model of Duchenne Muscular Dystrophy
- PMID: 33397205
- PMCID: PMC8063270
- DOI: 10.1089/hum.2019.255
Dose-Escalation Study of Systemically Delivered rAAVrh74.MHCK7.micro-dystrophin in the mdx Mouse Model of Duchenne Muscular Dystrophy
Abstract
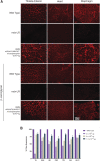
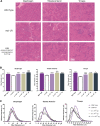
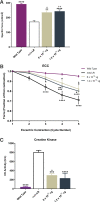
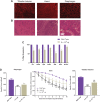
Duchenne muscular dystrophy (DMD) is a rare, X-linked, fatal, degenerative neuromuscular disease caused by mutations in the DMD gene. More than 2,000 mutations of the DMD gene are responsible for progressive loss of muscle strength, loss of ambulation, and generally respiratory and cardiac failure by age 30. Recently, gene transfer therapy has received widespread interest as a disease-modifying treatment for all patients with DMD. We designed an adeno-associated virus vector (rAAVrh74) containing a codon-optimized human micro-dystrophin transgene driven by a skeletal and cardiac muscle-specific promoter, MHCK7. To test the efficacy of rAAVrh74.MHCK7.micro-dystrophin, we evaluated systemic injections in mdx (dystrophin-null) mice at low (2 × 1012 vector genome [vg] total dose, 8 × 1013 vg/kg), intermediate (6 × 1012 vg total dose, 2 × 1014 vg/kg), and high doses (1.2 × 1013 vg total dose, 6 × 1014 vg/kg). Three months posttreatment, specific force increased in the diaphragm (DIA) and tibialis anterior muscle, with intermediate and high doses eliciting force outputs at wild-type (WT) levels. Histological improvement included reductions in fibrosis and normalization of myofiber size, specifically in the DIA, where results for low and intermediate doses were not significantly different from the WT. Significant reduction in central nucleation was also observed, although complete normalization to WT was not seen. No vector-associated toxicity was reported either by clinical or organ-specific laboratory assessments or following formal histopathology. The findings in this preclinical study provided proof of principle for safety and efficacy of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin at high vector titers, supporting initiation of a Phase I/II safety study in boys with DMD.
Keywords: AAV; Duchenne muscular dystrophy; dose-escalation; gene therapy; mdx mouse model; micro-dystrophin; rAAVrh74.MHCK7.micro-dystrophin.
Conflict of interest statement
R.A.P. is an employee of Sarepta Therapeutics, Inc., D.A.G. is an employee of Sarepta Therapeutics, Inc., K.N.H. and E.K.C.: No competing financial interest exists. E.L.P. is an employee of Sarepta Therapeutics, Inc., J.R.M. is the coinventor of the rAAVrh74.micro-dystrophin technology. This technology has been exclusively licensed to Sarepta Therapeutics, Inc., L.R.R.-K. is the coinventor of the AAVrh74.micro-dystrophin technology and eligible to receive financial consideration as a result. L.R.R.-K. is an employee of Sarepta Therapeutics, Inc.
Figures


References
-
- Emery AE. Population frequencies of inherited neuromuscular disease—a world survey. Neuromuscul Disord 1991;1:19–29 - PubMed
-
- Mendell JR, Lloyd-Puryear M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle Nerve 2013;48:21–26 - PubMed
-
- Mendell JR, Shilling C, Leslie ND, et al. Evidence based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol 2012;71:304–313 - PubMed
-
- Oudet C, Hanauer A, Clemens P, et al. Two hot spots of recombination in the DMD gene correlate with the deletion prone regions. Hum Mol Genet 1992;1:599–603 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
