Mesenchymal stromal cells to fight SARS-CoV-2: Taking advantage of a pleiotropic therapy
- PMID: 33397585
- PMCID: PMC7836230
- DOI: 10.1016/j.cytogfr.2020.12.002
Mesenchymal stromal cells to fight SARS-CoV-2: Taking advantage of a pleiotropic therapy
Abstract
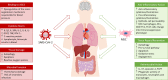
The devastating global impact of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has prompted scientists to develop novel strategies to fight Coronavirus Disease of 2019 (COVID-19), including the examination of pre-existing treatments for other viral infections in COVID-19 patients. This review provides a reasoned discussion of the possible use of Mesenchymal Stromal Cells (MSC) or their products as a treatment in SARS-CoV-2-infected patients. The main benefits and concerns of using this cellular therapy, guided by preclinical and clinical data obtained from similar pathologies will be reviewed. MSC represent a highly immunomodulatory cell population and their use may be safe according to clinical studies developed in other pathologies. Notably, four clinical trials and four case reports that have already been performed in COVID-19 patients obtained promising results. The clinical application of MSC in COVID-19 is very preliminary and further investigational studies are required to determine the efficacy of the MSC therapy. Nevertheless, these preliminary studies were important to understand the therapeutic potential of MSC in COVID-19. Based on these encouraging results, the United States Food and Drug Administration (FDA) authorized the compassionate use of MSC, but only in patients with Acute Respiratory Distress Syndrome (ARDS) and a poor prognosis. In fact, patients with severe SARS-CoV-2 can present infection and tissue damage in different organs, such as lung, heart, liver, kidney, gut and brain, affecting their function. MSC may have pleiotropic activities in COVID-19, with the capacity to fight inflammation and repair lesions in several organs.
Keywords: COVID-19; Cytokine storm; Cytokines/chemokines; Mesenchymal stromal/stem cells; Pleiotropic therapy; SARS-CoV-2.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
Figures
References
-
- Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Li X., Zhao Y., Yin K., He X., Gao Z., Wang Y., Yang B., Jin R., Stambler I., Lim L.W., Su H., Moskalev A., Cano A., Chakrabarti S., Min K.J., Ellison-Hughes G., Caruso C., Jin K., Zhao R.C. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

