Photoreceptive Ganglion Cells Drive Circuits for Local Inhibition in the Mouse Retina
- PMID: 33397711
- PMCID: PMC7896016
- DOI: 10.1523/JNEUROSCI.0674-20.2020
Photoreceptive Ganglion Cells Drive Circuits for Local Inhibition in the Mouse Retina
Abstract
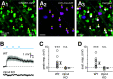
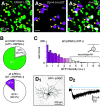
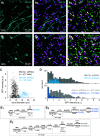
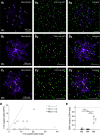
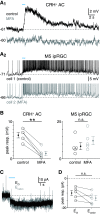
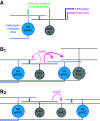
Intrinsically photosensitive retinal ganglion cells (ipRGCs) exhibit melanopsin-dependent light responses that persist in the absence of rod and cone photoreceptor-mediated input. In addition to signaling anterogradely to the brain, ipRGCs signal retrogradely to intraretinal circuitry via gap junction-mediated electrical synapses with amacrine cells (ACs). However, the targets and functions of these intraretinal signals remain largely unknown. Here, in mice of both sexes, we identify circuitry that enables M5 ipRGCs to locally inhibit retinal neurons via electrical synapses with a nonspiking GABAergic AC. During pharmacological blockade of rod- and cone-mediated input, whole-cell recordings of corticotropin-releasing hormone-expressing (CRH+) ACs reveal persistent visual responses that require both melanopsin expression and gap junctions. In the developing retina, ipRGC-mediated input to CRH+ ACs is weak or absent before eye opening, indicating a primary role for this input in the mature retina (i.e., in parallel with rod- and cone-mediated input). Among several ipRGC types, only M5 ipRGCs exhibit consistent anatomical and physiological coupling to CRH+ ACs. Optogenetic stimulation of local CRH+ ACs directly drives IPSCs in M4 and M5, but not M1-M3, ipRGCs. CRH+ ACs also inhibit M2 ipRGC-coupled spiking ACs, demonstrating direct interaction between discrete networks of ipRGC-coupled interneurons. Together, these results demonstrate a functional role for electrical synapses in translating ipRGC activity into feedforward and feedback inhibition of local retinal circuits.SIGNIFICANCE STATEMENT Melanopsin directly generates light responses in intrinsically photosensitive retinal ganglion cells (ipRGCs). Through gap junction-mediated electrical synapses with retinal interneurons, these uniquely photoreceptive RGCs may also influence the activity and output of neuronal circuits within the retina. Here, we identified and studied an electrical synaptic circuit that, in principle, could couple ipRGC activity to the chemical output of an identified retinal interneuron. Specifically, we found that M5 ipRGCs form electrical synapses with corticotropin-releasing hormone-expressing amacrine cells, which locally release GABA to inhibit specific RGC types. Thus, ipRGCs are poised to influence the output of diverse retinal circuits via electrical synapses with interneurons.
Keywords: amacrine cell; corticotropin releasing hormone; gap junction; ipRGC; melanopsin; retinal ganglion cell.
Copyright © 2021 the authors.
Figures






Similar articles
-
Structure and function of the gap junctional network of photoreceptive ganglion cells.Vis Neurosci. 2021 Sep 16;38:E014. doi: 10.1017/S0952523821000134. Vis Neurosci. 2021. PMID: 34652269 Free PMC article.
-
Retinal Waves Modulate an Intraretinal Circuit of Intrinsically Photosensitive Retinal Ganglion Cells.J Neurosci. 2016 Jun 29;36(26):6892-905. doi: 10.1523/JNEUROSCI.0572-16.2016. J Neurosci. 2016. PMID: 27358448 Free PMC article.
-
M1 ipRGCs Influence Visual Function through Retrograde Signaling in the Retina.J Neurosci. 2016 Jul 6;36(27):7184-97. doi: 10.1523/JNEUROSCI.3500-15.2016. J Neurosci. 2016. PMID: 27383593 Free PMC article.
-
Intrinsically photosensitive retinal ganglion cells.Sci China Life Sci. 2010 Jan;53(1):58-67. doi: 10.1007/s11427-010-0024-5. Epub 2010 Feb 12. Sci China Life Sci. 2010. PMID: 20596956 Review.
-
Intrinsically photosensitive retinal ganglion cells.Rev Physiol Biochem Pharmacol. 2012;162:59-90. doi: 10.1007/112_2011_4. Rev Physiol Biochem Pharmacol. 2012. PMID: 22160822 Review.
Cited by
-
Structure and function of the gap junctional network of photoreceptive ganglion cells.Vis Neurosci. 2021 Sep 16;38:E014. doi: 10.1017/S0952523821000134. Vis Neurosci. 2021. PMID: 34652269 Free PMC article.
-
Optic nerve injury impairs intrinsic mechanisms underlying electrical activity in a resilient retinal ganglion cell.J Physiol. 2025 Feb 22:10.1113/JP286414. doi: 10.1113/JP286414. Online ahead of print. J Physiol. 2025. PMID: 39985791
-
Specific retinal neurons regulate context-dependent defensive responses to visual threat.PNAS Nexus. 2024 Sep 24;3(10):pgae423. doi: 10.1093/pnasnexus/pgae423. eCollection 2024 Oct. PNAS Nexus. 2024. PMID: 39359403 Free PMC article.
-
Burning the candle at both ends: Intraretinal signaling of intrinsically photosensitive retinal ganglion cells.Front Cell Neurosci. 2023 Jan 6;16:1095787. doi: 10.3389/fncel.2022.1095787. eCollection 2022. Front Cell Neurosci. 2023. PMID: 36687522 Free PMC article. Review.
-
The Retinal Basis of Light Aversion in Neonatal Mice.J Neurosci. 2022 May 18;42(20):4101-4115. doi: 10.1523/JNEUROSCI.0151-22.2022. Epub 2022 Apr 8. J Neurosci. 2022. PMID: 35396331 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
