Small-molecule inhibitors for the Prp8 intein as antifungal agents
- PMID: 33397721
- PMCID: PMC7812778
- DOI: 10.1073/pnas.2008815118
Small-molecule inhibitors for the Prp8 intein as antifungal agents
Abstract
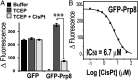
Self-splicing proteins, called inteins, are present in many human pathogens, including the emerging fungal threats Cryptococcus neoformans (Cne) and Cryptococcus gattii (Cga), the causative agents of cryptococcosis. Inhibition of protein splicing in Cryptococcus sp. interferes with activity of the only intein-containing protein, Prp8, an essential intron splicing factor. Here, we screened a small-molecule library to find addititonal, potent inhibitors of the Cne Prp8 intein using a split-GFP splicing assay. This revealed the compound 6G-318S, with IC50 values in the low micromolar range in the split-GFP assay and in a complementary split-luciferase system. A fluoride derivative of the compound 6G-318S displayed improved cytotoxicity in human lung carcinoma cells, although there was a slight reduction in the inhibition of splicing. 6G-318S and its derivative inhibited splicing of the Cne Prp8 intein in vivo in Escherichia coli and in C. neoformans Moreover, the compounds repressed growth of WT C. neoformans and C. gattii In contrast, the inhibitors were less potent at inhibiting growth of the inteinless Candida albicans Drug resistance was observed when the Prp8 intein was overexpressed in C. neoformans, indicating specificity of this molecule toward the target. No off-target activity was observed, such as inhibition of serine/cysteine proteases. The inhibitors bound covalently to the Prp8 intein and binding was reduced when the active-site residue Cys1 was mutated. 6G-318S showed a synergistic effect with amphotericin B and additive to indifferent effects with a few other clinically used antimycotics. Overall, the identification of these small-molecule intein-splicing inhibitors opens up prospects for a new class of antifungals.
Keywords: Cryptococcus; Prp8 intein; antifungal; protein splicing; small-molecule inhibitor.
Conflict of interest statement
The authors declare no competing interest.
Figures





Similar articles
-
Calcimycin Inhibits Cryptococcus neoformans In Vitro and In Vivo by Targeting the Prp8 Intein Splicing.ACS Infect Dis. 2022 Sep 9;8(9):1851-1868. doi: 10.1021/acsinfecdis.2c00137. Epub 2022 Aug 10. ACS Infect Dis. 2022. PMID: 35948057 Free PMC article.
-
Cisplatin protects mice from challenge of Cryptococcus neoformans by targeting the Prp8 intein.Emerg Microbes Infect. 2019;8(1):895-908. doi: 10.1080/22221751.2019.1625727. Emerg Microbes Infect. 2019. PMID: 31223062 Free PMC article.
-
Effect of insertion of intein to Cryptococcus amylolentus, a nonpathogenic fungus closely related to causative agents of cryptococcosis.Microb Pathog. 2025 Feb;199:107267. doi: 10.1016/j.micpath.2024.107267. Epub 2024 Dec 28. Microb Pathog. 2025. PMID: 39736341
-
Inteins as Drug Targets and Therapeutic Tools.Front Mol Biosci. 2022 Feb 8;9:821146. doi: 10.3389/fmolb.2022.821146. eCollection 2022. Front Mol Biosci. 2022. PMID: 35211511 Free PMC article. Review.
-
[Protein splicing and its application].Sheng Wu Gong Cheng Xue Bao. 2003 Mar;19(2):249-54. Sheng Wu Gong Cheng Xue Bao. 2003. PMID: 15966332 Review. Chinese.
Cited by
-
Cryptococcus neoformans Prp8 Intein: An In Vivo Target-Based Drug Screening System in Saccharomyces cerevisiae to Identify Protein Splicing Inhibitors and Explore Its Dynamics.J Fungi (Basel). 2022 Aug 12;8(8):846. doi: 10.3390/jof8080846. J Fungi (Basel). 2022. PMID: 36012834 Free PMC article.
-
A universal fluorescence polarization high throughput screening assay to target the SAM-binding sites of SARS-CoV-2 and other viral methyltransferases.Emerg Microbes Infect. 2023 Dec;12(1):2204164. doi: 10.1080/22221751.2023.2204164. Emerg Microbes Infect. 2023. PMID: 37060263 Free PMC article.
-
Inteins-mechanism of protein splicing, emerging regulatory roles, and applications in protein engineering.Front Microbiol. 2023 Nov 8;14:1305848. doi: 10.3389/fmicb.2023.1305848. eCollection 2023. Front Microbiol. 2023. PMID: 38029209 Free PMC article. Review.
-
Gene drive that results in addiction to a temperature-sensitive version of an essential gene triggers population collapse in Drosophila.Proc Natl Acad Sci U S A. 2021 Dec 7;118(49):e2107413118. doi: 10.1073/pnas.2107413118. Proc Natl Acad Sci U S A. 2021. PMID: 34845012 Free PMC article.
-
Monitoring Protein Stability In Vivo Using an Intein-Based Biosensor.Bio Protoc. 2025 Apr 20;15(8):e5271. doi: 10.21769/BioProtoc.5271. eCollection 2025 Apr 20. Bio Protoc. 2025. PMID: 40291413 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

