Afferent Lymphatic Transport and Peripheral Tissue Immunity
- PMID: 33397740
- PMCID: PMC7789254
- DOI: 10.4049/jimmunol.2001060
Afferent Lymphatic Transport and Peripheral Tissue Immunity
Abstract
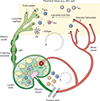
Lymphatic vessels provide an anatomical framework for immune surveillance and adaptive immune responses. Although appreciated as the route for Ag and dendritic cell transport, peripheral lymphatic vessels are often not considered active players in immune surveillance. Lymphatic vessels, however, integrate contextual cues that directly regulate transport, including changes in intrinsic pumping and capillary remodeling, and express a dynamic repertoire of inflammatory chemokines and adhesion molecules that facilitates leukocyte egress out of inflamed tissue. These mechanisms together contribute to the course of peripheral tissue immunity. In this review, we focus on context-dependent mechanisms that regulate fluid and cellular transport out of peripheral nonlymphoid tissues to provide a framework for understanding the effects of afferent lymphatic transport on immune surveillance, peripheral tissue inflammation, and adaptive immunity.
Copyright © 2021 by The American Association of Immunologists, Inc.
Figures

References
-
- Petrova TV, and Koh GY. 2020. Biological functions of lymphatic vessels. Science 369: eaax4063. - PubMed
-
- Santambrogio L 2018. Lymph Formation and Composition. Lymphedema 139–152.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

