Analysis of SARS-CoV-2 antibodies in COVID-19 convalescent blood using a coronavirus antigen microarray
- PMID: 33397903
- PMCID: PMC7782488
- DOI: 10.1038/s41467-020-20095-2
Analysis of SARS-CoV-2 antibodies in COVID-19 convalescent blood using a coronavirus antigen microarray
Abstract
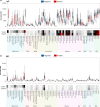
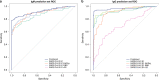
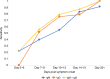
The current practice for diagnosis of COVID-19, based on SARS-CoV-2 PCR testing of pharyngeal or respiratory specimens in a symptomatic patient at high epidemiologic risk, likely underestimates the true prevalence of infection. Serologic methods can more accurately estimate the disease burden by detecting infections missed by the limited testing performed to date. Here, we describe the validation of a coronavirus antigen microarray containing immunologically significant antigens from SARS-CoV-2, in addition to SARS-CoV, MERS-CoV, common human coronavirus strains, and other common respiratory viruses. A comparison of antibody profiles detected on the array from control sera collected prior to the SARS-CoV-2 pandemic versus convalescent blood specimens from virologically confirmed COVID-19 cases demonstrates near complete discrimination of these two groups, with improved performance from use of antigen combinations that include both spike protein and nucleoprotein. This array can be used as a diagnostic tool, as an epidemiologic tool to more accurately estimate the disease burden of COVID-19, and as a research tool to correlate antibody responses with clinical outcomes.
Conflict of interest statement
The coronavirus antigen microarray is the intellectual property of the Regents of the University of California that is licensed for commercialization to Nanommune Inc. (Irvine, CA), a private company for which P. Felgner is the largest shareholder and several co-authors (R. de Assis, A. Jain, R. Nakajima, A. Jasinskas, J. Obiero, Jiin Felgner, H. Davies, and S. Khan) also own shares. Nanommune Inc. has a business partnership with Sino Biological Inc. (Beijing, China) which expressed and purified the antigens used in this study. The convalescent plasma used in this study was collected for clinical use by independent blood centers using licensed plasma or platelet processing systems manufactured by Cerus Corporation, for which multiple authors (L. Corash, A. Bagri, and Johannes Irsch) are shareholders and employees. Convalescent sera were also provided by Ortho Clinical Diagnostics, which is using these specimens to validate a clinical diagnostic test, and P. Hosimer, C. Noesen, and P. Contestable are shareholders and employees of this company. M. Battegay, A. Buser, A. Holbro, Philip J. Norris, Mars Stone, Graham Simmons, Martin Schreiber, Oluwasanmi Adenaiye, Sheldon Tai, Filbert Hong, Donald K. Milton, Michael P. Busch, and the Prometheus-UMD consortium investigators have no conflicts of interest to disclose.
Figures

Update of
-
Analysis of SARS-CoV-2 Antibodies in COVID-19 Convalescent Blood using a Coronavirus Antigen Microarray.bioRxiv [Preprint]. 2020 May 8:2020.04.15.043364. doi: 10.1101/2020.04.15.043364. bioRxiv. 2020. Update in: Nat Commun. 2021 Jan 4;12(1):6. doi: 10.1038/s41467-020-20095-2. PMID: 32511302 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

