Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer
- PMID: 33397968
- PMCID: PMC7782714
- DOI: 10.1038/s41523-020-00208-2
Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer
Erratum in
-
Author Correction: Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer.NPJ Breast Cancer. 2023 Apr 29;9(1):32. doi: 10.1038/s41523-023-00538-x. NPJ Breast Cancer. 2023. PMID: 37120452 Free PMC article. No abstract available.
Abstract
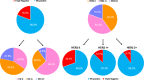
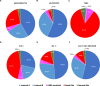
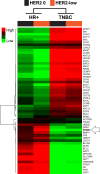
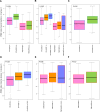
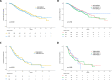
Novel antibody-drug conjugates against HER2 are showing high activity in HER2-negative breast cancer (BC) with low HER2 expression (i.e., 1+ or 2+ and lack of ERBB2 amplification). However, the clinical and molecular features of HER2-low BC are yet to be elucidated. Here, we collected retrospective clinicopathological and PAM50 data from 3,689 patients with HER2-negative disease and made the following observations. First, the proportion of HER2-low was higher in HR-positive disease (65.4%) than triple-negative BC (TNBC, 36.6%). Second, within HR-positive disease, ERBB2 and luminal-related genes were more expressed in HER2-low than HER2 0. In contrast, no gene was found differentially expressed in TNBC according to HER2 expression. Third, within HER2-low, ERBB2 levels were higher in HR-positive disease than TNBC. Fourth, HER2-low was not associated with overall survival in HR-positive disease and TNBC. Finally, the reproducibility of HER2-low among pathologists was suboptimal. This study emphasizes the large biological heterogeneity of HER2-low BC, and the need to implement reproducible and sensitive assays to measure low HER2 expression.
Conflict of interest statement
A.P. has declared an immediate family member being employed by Novartis, personal honoraria from Pfizer, Novartis, Roche, MSD Oncology, Lilly and Daiichi Sankyo, travel, accommodations and expenses paid by Daiichi Sankyo, research funding from Roche and Novartis, consulting/advisory role for NanoString Technologies, Amgen, Roche, Novartis, Pfizer and Bristol-Myers Squibb, and patent PCT/EP2016/080056: HER2 AS A PREDICTOR OF RESPONSE TO DUAL HER2 BLOCKADE IN THE ABSENCE OF CYTOTOXIC THERAPY. C.B. declares research funding, consulting and honoraria form Astra Zeneca, Novartis, Roche, GSK, Pfizer, Libbs, Daiichi Sankyo, and MSD. A.L. declares clinical research fundings from Amgen, Astra Zeneca, Boehringer-Ingelheim, GSK, Novartis, Pfizer, Roche/Genentech, Eisai, Celgene, Pierre Fabre and advisory boards and consulting for Novartis, Pfizer, Roche/Genentech, Eisai, Celgene. M.M. declares research grants from Roche, PUMA and Novartis, consulting/advisory fees from AstraZeneca, Amgen, Taiho Oncology, Roche/Genentech, Novartis, PharmaMar, Eli Lilly, PUMA, Taiho Oncology, Daiichi Sankyo and Pfizer and speakers’ honoraria from AstraZeneca, Amgen, Roche/Genentech, Novartis and Pfizer. J.G. has declared speakers’ honoraria and participation in advisory boards from Pfizer, Roche, and Novartis. S.D.P. has declared honoraria from Roche, Pfizer, Astra-Zeneca, Novartis, Celgene, Eli Lilly, Amgen, and Eisai. The other authors have nothing to declare.
Figures
References
-
- Wolff AC, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. - DOI - PubMed
-
- Wolff AC, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. - DOI - PubMed
-
- Schalper KA, Kumar S, Hui P, Rimm DL, Gershkovich P. A retrospective population-based comparison of HER2 immunohistochemistry and fluorescence in situ hybridization in breast carcinomas: impact of 2007 American Society of Clinical Oncology/College of American Pathologists criteria. Arch. Pathol. Lab. Med. 2014;138:213–219. doi: 10.5858/arpa.2012-0617-OA. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

