Mitochondrial health is enhanced in rats with higher vs. lower intrinsic exercise capacity and extended lifespan
- PMID: 33398019
- PMCID: PMC7782588
- DOI: 10.1038/s41514-020-00054-3
Mitochondrial health is enhanced in rats with higher vs. lower intrinsic exercise capacity and extended lifespan
Abstract
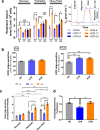
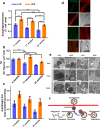
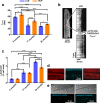
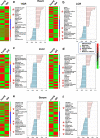
The intrinsic aerobic capacity of an organism is thought to play a role in aging and longevity. Maximal respiratory rate capacity, a metabolic performance measure, is one of the best predictors of cardiovascular- and all-cause mortality. Rats selectively bred for high-(HCR) vs. low-(LCR) intrinsic running-endurance capacity have up to 31% longer lifespan. We found that positive changes in indices of mitochondrial health in cardiomyocytes (respiratory reserve, maximal respiratory capacity, resistance to mitochondrial permeability transition, autophagy/mitophagy, and higher lipids-over-glucose utilization) are uniformly associated with the extended longevity in HCR vs. LCR female rats. Cross-sectional heart metabolomics revealed pathways from lipid metabolism in the heart, which were significantly enriched by a select group of strain-dependent metabolites, consistent with enhanced lipids utilization by HCR cardiomyocytes. Heart-liver-serum metabolomics further revealed shunting of lipidic substrates between the liver and heart via serum during aging. Thus, mitochondrial health in cardiomyocytes is associated with extended longevity in rats with higher intrinsic exercise capacity and, probably, these findings can be translated to other populations as predictors of outcomes of health and survival.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hill AV. The physiological basis of athletic records. Nature. 1925;116:544–548. doi: 10.1038/116544a0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

