miR-379 deletion ameliorates features of diabetic kidney disease by enhancing adaptive mitophagy via FIS1
- PMID: 33398021
- PMCID: PMC7782535
- DOI: 10.1038/s42003-020-01516-w
miR-379 deletion ameliorates features of diabetic kidney disease by enhancing adaptive mitophagy via FIS1
Erratum in
-
Publisher Correction: miR-379 deletion ameliorates features of diabetic kidney disease by enhancing adaptive mitophagy via FIS1.Commun Biol. 2021 Feb 4;4(1):175. doi: 10.1038/s42003-021-01691-4. Commun Biol. 2021. PMID: 33542531 Free PMC article. No abstract available.
Abstract
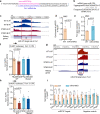
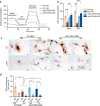
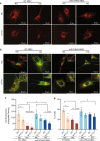
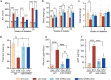
Diabetic kidney disease (DKD) is a major complication of diabetes. Expression of members of the microRNA (miRNA) miR-379 cluster is increased in DKD. miR-379, the most upstream 5'-miRNA in the cluster, functions in endoplasmic reticulum (ER) stress by targeting EDEM3. However, the in vivo functions of miR-379 remain unclear. We created miR-379 knockout (KO) mice using CRISPR-Cas9 nickase and dual guide RNA technique and characterized their phenotype in diabetes. We screened for miR-379 targets in renal mesangial cells from WT vs. miR-379KO mice using AGO2-immunopreciptation and CLASH (cross-linking, ligation, sequencing hybrids) and identified the redox protein thioredoxin and mitochondrial fission-1 protein. miR-379KO mice were protected from features of DKD as well as body weight loss associated with mitochondrial dysfunction, ER- and oxidative stress. These results reveal a role for miR-379 in DKD and metabolic processes via reducing adaptive mitophagy. Strategies targeting miR-379 could offer therapeutic options for DKD.
Conflict of interest statement
The authors declare no competing interests.
Figures

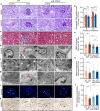
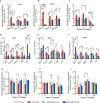
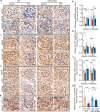
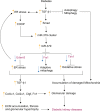
Comment in
-
Promises and challenges of miRNA therapeutics.Am J Physiol Renal Physiol. 2022 Dec 1;323(6):F673-F674. doi: 10.1152/ajprenal.00251.2022. Epub 2022 Oct 20. Am J Physiol Renal Physiol. 2022. PMID: 36264885 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

