Dose-dependent response to infection with SARS-CoV-2 in the ferret model and evidence of protective immunity
- PMID: 33398055
- PMCID: PMC7782478
- DOI: 10.1038/s41467-020-20439-y
Dose-dependent response to infection with SARS-CoV-2 in the ferret model and evidence of protective immunity
Abstract
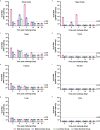
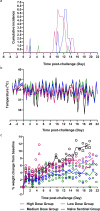
There is a vital need for authentic COVID-19 animal models to enable the pre-clinical evaluation of candidate vaccines and therapeutics. Here we report a dose titration study of SARS-CoV-2 in the ferret model. After a high (5 × 106 pfu) and medium (5 × 104 pfu) dose of virus is delivered, intranasally, viral RNA shedding in the upper respiratory tract (URT) is observed in 6/6 animals, however, only 1/6 ferrets show similar signs after low dose (5 × 102 pfu) challenge. Following sequential culls pathological signs of mild multifocal bronchopneumonia in approximately 5-15% of the lung is seen on day 3, in high and medium dosed groups. Ferrets re-challenged, after virus shedding ceased, are fully protected from acute lung pathology. The endpoints of URT viral RNA replication & distinct lung pathology are observed most consistently in the high dose group. This ferret model of SARS-CoV-2 infection presents a mild clinical disease.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/ (WHO, 2020).
-
- WHO. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mis... (WHO, 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

