Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer's disease
- PMID: 33398092
- PMCID: PMC8166828
- DOI: 10.1038/s41418-020-00685-9
Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer's disease
Erratum in
-
Correction: Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer's disease.Cell Death Differ. 2024 Aug;31(8):1099. doi: 10.1038/s41418-024-01290-w. Cell Death Differ. 2024. PMID: 38575681 Free PMC article. No abstract available.
Abstract
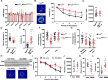
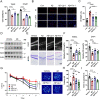
Iron homeostasis disturbance has been implicated in Alzheimer's disease (AD), and excess iron exacerbates oxidative damage and cognitive defects. Ferroptosis is a nonapoptotic form of cell death dependent upon intracellular iron. However, the involvement of ferroptosis in the pathogenesis of AD remains elusive. Here, we report that ferroportin1 (Fpn), the only identified mammalian nonheme iron exporter, was downregulated in the brains of APPswe/PS1dE9 mice as an Alzheimer's mouse model and Alzheimer's patients. Genetic deletion of Fpn in principal neurons of the neocortex and hippocampus by breeding Fpnfl/fl mice with NEX-Cre mice led to AD-like hippocampal atrophy and memory deficits. Interestingly, the canonical morphological and molecular characteristics of ferroptosis were observed in both Fpnfl/fl/NEXcre and AD mice. Gene set enrichment analysis (GSEA) of ferroptosis-related RNA-seq data showed that the differentially expressed genes were highly enriched in gene sets associated with AD. Furthermore, administration of specific inhibitors of ferroptosis effectively reduced the neuronal death and memory impairments induced by Aβ aggregation in vitro and in vivo. In addition, restoring Fpn ameliorated ferroptosis and memory impairment in APPswe/PS1dE9 mice. Our study demonstrates the critical role of Fpn and ferroptosis in the progression of AD, thus provides promising therapeutic approaches for this disease.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures





References
Publication types
MeSH terms
Grants and funding
- 81700789/National Natural Science Foundation of China (National Science Foundation of China)
- 81701653/National Natural Science Foundation of China (National Science Foundation of China)
- 31330036/National Natural Science Foundation of China (National Science Foundation of China)
- 31530034/National Natural Science Foundation of China (National Science Foundation of China)
- 31721002/National Natural Science Foundation of China (National Science Foundation of China)
- 81871108/National Natural Science Foundation of China (National Science Foundation of China)
- 82030032/National Natural Science Foundation of China (National Science Foundation of China)
- 81829002/National Natural Science Foundation of China (National Science Foundation of China)
- 81961128005/National Natural Science Foundation of China (National Science Foundation of China)
- 2017M612467/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

