Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination
- PMID: 33398162
- PMCID: PMC8095049
- DOI: 10.1038/s41591-020-1131-x
Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination
Abstract
Metastasis is the primary cause of cancer mortality, and cancer frequently metastasizes to the liver. It is not clear whether liver immune tolerance mechanisms contribute to cancer outcomes. We report that liver metastases diminish immunotherapy efficacy systemically in patients and preclinical models. Patients with liver metastases derive limited benefit from immunotherapy independent of other established biomarkers of response. In multiple mouse models, we show that liver metastases siphon activated CD8+ T cells from systemic circulation. Within the liver, activated antigen-specific Fas+CD8+ T cells undergo apoptosis following their interaction with FasL+CD11b+F4/80+ monocyte-derived macrophages. Consequently, liver metastases create a systemic immune desert in preclinical models. Similarly, patients with liver metastases have reduced peripheral T cell numbers and diminished tumoral T cell diversity and function. In preclinical models, liver-directed radiotherapy eliminates immunosuppressive hepatic macrophages, increases hepatic T cell survival and reduces hepatic siphoning of T cells. Thus, liver metastases co-opt host peripheral tolerance mechanisms to cause acquired immunotherapy resistance through CD8+ T cell deletion, and the combination of liver-directed radiotherapy and immunotherapy could promote systemic antitumor immunity.
Conflict of interest statement
Competing interests
J.Y., M.D.G., S.L., Y.S., S.N.J., J.E.C., S.M.R., J.J.W., X.L., Z.C., J.Z., Y.B., L.J., A.T., J.S., R.K.A., M.S., B.S.R., F.S., S.P.N., X.C., S.W., W.S., L.V., C.M., M.A.M., C.A.S., K.C., I.K., V.T.M., T.S.L., N.R., F.W., A.M.C. and M.C. report no conflicts. A.Q. has research funding from Merck and Clovis. I.E.N. serves as a consultant for Endectra. A.A. serves as a consultant for Merck, AstraZeneca, Bristol-Myers Squibb and Pfizer/EMD Serono. A.A. receives research funding through the University of Michigan from Merck, Genentech, Prometheus Laboratories, Mirati Therapeutics, Roche, Bayer, Progenics, Astellas Pharma, Arcus Biosciences, AstraZeneca, Bristol-Myers Squibb and Clovis Oncology. C.D.L. serves as a consultant for Immunocore. C.D.L receives travel, accommodations and expenses from Bristol-Myers Squibb and Immunocore. C.D.L. receives research funding from Bristol-Myers Squibb, Merck, Novartis and Dynavax. W.Z. has served as a scientific advisor for Cstone, Oncopia and Hengenix.
Figures









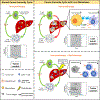
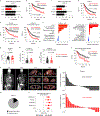


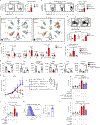


Comment in
-
Liver metastases inhibit immunotherapy efficacy.Nat Med. 2021 Jan;27(1):25-27. doi: 10.1038/s41591-020-01190-9. Nat Med. 2021. PMID: 33442002 No abstract available.
-
Liver metastases siphon T cells and blunt immunotherapy responses.Nat Rev Gastroenterol Hepatol. 2021 Mar;18(3):150. doi: 10.1038/s41575-021-00421-9. Nat Rev Gastroenterol Hepatol. 2021. PMID: 33495586 No abstract available.
-
Liver metastases cultivate an immune desert.Nat Rev Cancer. 2021 Mar;21(3):143. doi: 10.1038/s41568-021-00338-0. Nat Rev Cancer. 2021. PMID: 33547456 No abstract available.
-
Liver Metastasis Irradiation Can Restore Immunotherapeutic Responsiveness.Trends Immunol. 2021 Apr;42(4):275-277. doi: 10.1016/j.it.2021.02.007. Epub 2021 Feb 23. Trends Immunol. 2021. PMID: 33637449
-
Cancer immunotherapy: Macs in the middle.Immunity. 2021 Mar 9;54(3):409-411. doi: 10.1016/j.immuni.2021.02.012. Immunity. 2021. PMID: 33691132
-
Liver metastases "siphon" off immunotherapy response.Hepatobiliary Surg Nutr. 2021 Aug;10(4):526-529. doi: 10.21037/hbsn-21-215. Hepatobiliary Surg Nutr. 2021. PMID: 34430535 Free PMC article. No abstract available.
References
-
- Mehlen P & Puisieux A Metastasis: a question of life or death. Nat. Rev. Cancer 6, 449–458 (2006). - PubMed
-
- Disibio G & French SW Metastatic patterns of cancers: results from a large autopsy study. Arch. Pathol. Lab. Med. 132, 931–939 (2008). - PubMed
-
- Doherty DG Immunity, tolerance and autoimmunity in the liver: a comprehensive review. J. Autoimmun. 66, 60–75 (2016). - PubMed
-
- Crispe IN Hepatic T cells and liver tolerance. Nat. Rev. Immunol. 3, 51–62 (2003). - PubMed
Publication types
MeSH terms
Grants and funding
- P30CA46592/U-M | Comprehensive Cancer Center, University of Michigan (U-M Comprehensive Cancer Center)/International
- UM1 HG006508/HG/NHGRI NIH HHS/United States
- 1UM1HG006508/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)/International
- S10 OD025046/OD/NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- S10OD020053/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)/International
- R01 CA217510/CA/NCI NIH HHS/United States
- R01 CA240515/CA/NCI NIH HHS/United States
- R01 CA248430/CA/NCI NIH HHS/United States
- CA233487/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)/International
- R01 CA123088/CA/NCI NIH HHS/United States
- CA240515/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)/International
- U01CA216440/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)/International
- R01 CA099985/CA/NCI NIH HHS/United States
- U01 CA216449/CA/NCI NIH HHS/United States
- CA248430, CA217648, CA123088, CA099985, CA193136, CA152470/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)/International
- R01 CA193136/CA/NCI NIH HHS/United States
- R01 CA233487/CA/NCI NIH HHS/United States
- S10 OD020053/OD/NIH HHS/United States
- R01 CA152470/CA/NCI NIH HHS/United States
- R01 CA214911/CA/NCI NIH HHS/United States
- T32 GM007863/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

