Structure-Based Virtual Screening and Biochemical Validation to Discover a Potential Inhibitor of the SARS-CoV-2 Main Protease
- PMID: 33398250
- PMCID: PMC7754785
- DOI: 10.1021/acsomega.0c04808
Structure-Based Virtual Screening and Biochemical Validation to Discover a Potential Inhibitor of the SARS-CoV-2 Main Protease
Abstract
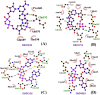
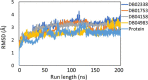
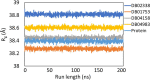
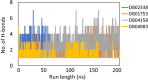
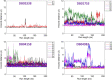
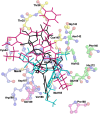
The recent pandemic caused by SARS-CoV-2 has led the world to a standstill, causing a medical and economic crisis worldwide. This crisis has triggered an urgent need to discover a possible treatment strategy against this novel virus using already-approved drugs. The main protease (Mpro) of this virus plays a critical role in cleaving the translated polypeptides that makes it a potential drug target against COVID-19. Taking advantage of the recently discovered three-dimensional structure of Mpro, we screened approved drugs from the Drug Bank to find a possible inhibitor against Mpro using computational methods and further validating them with biochemical studies. The docking and molecular dynamics study revealed that DB04983 (denufosol) showed the best glide docking score, -11.884 kcal/mol, and MM-PBSA binding free energy, -10.96 kcal/mol. Cobicistat, cangrelor (previous computational studies in our lab), and denufosol (current study) were tested for the in vitro inhibitory effects on Mpro. The IC50 values of these drugs were ∼6.7 μM, 0.9 mM, and 1.3 mM, respectively, while the values of dissociation constants calculated using surface plasmon resonance were ∼2.1 μM, 0.7 mM, and 1.4 mM, respectively. We found that cobicistat is the most efficient inhibitor of Mpro both in silico and in vitro. In conclusion, cobicistat, which is already an FDA-approved drug being used against HIV, may serve as a good inhibitor against the main protease of SARS-CoV-2 that, in turn, can help in combating COVID-19, and these results can also form the basis for the rational structure-based drug design against COVID-19.
© 2020 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- CDC Coronavirus disease 2019 (COVID-19). Cent. Dis. Control Prev. 2020.
-
- Myint S. H. Human Coronaviruses: A Brief Review. Rev. Med. Virol. 1994, 4, 35–46. 10.1002/rmv.1980040108. - DOI
-
- Hui D. S.; Azhar E. I.; Madani T. A.; Ntoumi F.; Kock R.; Dar O.; Ippolito G.; Mchugh T. D.; Memish Z. A.; Drosten C.; Zumla A.; Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infec. Diseases 2020, 91, 264–266. 10.1016/j.ijid.2020.01.009. - DOI - PMC - PubMed
-
- Fan H. H.; Wang L. Q.; Liu W. L.; An X. P.; Liu Z. D.; He X. Q.; Song L. H.; Tong Y. G. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin. Med. J. 2020, 133, 1051–1056. 10.1097/CM9.0000000000000797. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

