A deafness-associated tRNA mutation caused pleiotropic effects on the m1G37 modification, processing, stability and aminoacylation of tRNAIle and mitochondrial translation
- PMID: 33398350
- PMCID: PMC7826259
- DOI: 10.1093/nar/gkaa1225
A deafness-associated tRNA mutation caused pleiotropic effects on the m1G37 modification, processing, stability and aminoacylation of tRNAIle and mitochondrial translation
Abstract
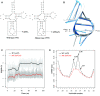
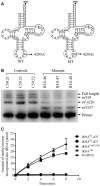
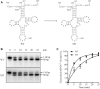
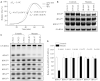
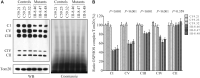
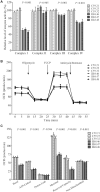
Defects in the posttranscriptional modifications of mitochondrial tRNAs have been linked to human diseases, but their pathophysiology remains elusive. In this report, we investigated the molecular mechanism underlying a deafness-associated tRNAIle 4295A>G mutation affecting a highly conserved adenosine at position 37, 3' adjacent to the tRNA's anticodon. Primer extension and methylation activity assays revealed that the m.4295A>G mutation introduced a tRNA methyltransferase 5 (TRMT5)-catalyzed m1G37 modification of tRNAIle. Molecular dynamics simulations suggested that the m.4295A>G mutation affected tRNAIle structure and function, supported by increased melting temperature, conformational changes and instability of mutated tRNA. An in vitro processing experiment revealed that the m.4295A>G mutation reduced the 5' end processing efficiency of tRNAIle precursors, catalyzed by RNase P. We demonstrated that cybrid cell lines carrying the m.4295A>G mutation exhibited significant alterations in aminoacylation and steady-state levels of tRNAIle. The aberrant tRNA metabolism resulted in the impairment of mitochondrial translation, respiratory deficiency, decreasing membrane potentials and ATP production, increasing production of reactive oxygen species and promoting autophagy. These demonstrated the pleiotropic effects of m.4295A>G mutation on tRNAIle and mitochondrial functions. Our findings highlighted the essential role of deficient posttranscriptional modifications in the structure and function of tRNA and their pathogenic consequence of deafness.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- El Yacoubi B., Bailly M., de Crécy-Lagard V.. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 2012; 46:69–95. - PubMed
-
- Suzuki T., Nagao A., Suzuki T.. Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu. Rev. Genet. 2011; 45:299–329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

