Melatonin inhibits proliferation and viability and promotes apoptosis in colorectal cancer cells via upregulation of the microRNA-34a/449a cluster
- PMID: 33398374
- PMCID: PMC7809902
- DOI: 10.3892/mmr.2021.11826
Melatonin inhibits proliferation and viability and promotes apoptosis in colorectal cancer cells via upregulation of the microRNA-34a/449a cluster
Abstract
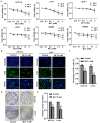
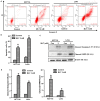
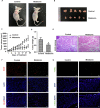
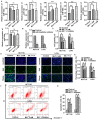
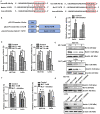
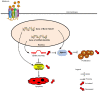
Colorectal cancer (CRC) has a significant burden on healthcare systems worldwide, and is associated with high morbidity and mortality rates in patients. In 2020, the estimated new cases of colon cancer in the United States are 78,300 in men and 69,650 in women. Thus, developing effective and novel alternative agents and adjuvants with reduced side effects is important to reduce the lethality of the disease and improve the quality of life of patients. Melatonin, a pineal hormone that possesses numerous physiological functions, including anti‑inflammatory and antitumor activities, can be found in various tissues, including the gastrointestinal tract. Melatonin exerts anticarcinogenic effects via various mechanisms; however, the identified underlying molecular mechanisms do not explain the full breadth of anti‑CRC effects mediated by melatonin. MicroRNAs (miRs) serve critical roles in tumorigenesis, however, whether melatonin can inhibit CRC by regulating miRs is not completely understood. In the present study, the roles and mechanism underlying melatonin in CRC were investigated. The proliferation of human CRC cells was tested by CCK8, EDU and colony formation assay. The apoptosis of cancer cells was detected by flow cytometry and western blotting. A xenograft mouse model was constructed and the proliferation and apoptosis of tumor tissue was detected by Ki‑67 and TUNEL staining assay respectively. Reverse transcription‑quantitative PCR and western blotting were performed to measure the regulation of miRs on mRNA, and the dual‑luciferase report analysis experiment was used to verify the direct target genes of miRs. Compared with the control group, melatonin inhibited viability and proliferation, and induced apoptosis in CRC cells. Additionally, the effect of melatonin in a xenograft mouse model was assessed. Compared with the control group, melatonin significantly enhanced the expression levels of the miR‑34a/449a cluster, reduced CRC cell proliferation and viability, and increased CRC cell apoptosis. Finally, the dual‑luciferase reporter assay indicated that Bcl‑2 and Notch1 were the target mRNAs of the miR‑34a/449a cluster. To the best of our knowledge, the present study was the first to suggest that melatonin inhibited proliferation and viability, and promoted apoptosis in CRC cells via upregulating the expression of the miR‑34a/449a cluster in vitro and in vivo. Therefore, melatonin may serve as a potential therapeutic for CRC.
Keywords: melatonin; colorectal cancer; microRNA‑34a/449a cluster; proliferation; apoptosis.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

