Loss of α7 nicotinic acetylcholine receptors in GABAergic neurons causes sex-dependent decreases in radial glia-like cell quantity and impairments in cognitive and social behavior
- PMID: 33398432
- PMCID: PMC8121181
- DOI: 10.1007/s00429-020-02179-3
Loss of α7 nicotinic acetylcholine receptors in GABAergic neurons causes sex-dependent decreases in radial glia-like cell quantity and impairments in cognitive and social behavior
Abstract
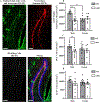
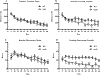
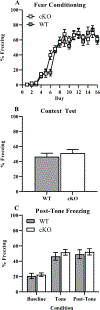
The dentate gyrus (DG) is a unique brain structure in that neurons can be generated postnatally and integrated within existing circuitry throughout life. The maturation process of these newly generated neurons (granule cells) is modulated by nicotinic acetylcholine receptors (nAChRs) through a variety of mechanisms such as neural stem pool proliferation, cell survival, signal modulation, and dendritic integration. Disrupted nAChR signaling has been implicated in neuropsychiatric and neurodegenerative disorders, potentially via alterations in DG neurogenesis. GABAergic interneurons are known to express nAChRs, predominantly the α7 subtype, and have been shown to shape development, integration, and circuit reorganization of DG granule cells. Therefore, we examined histological and behavioral effects of knocking out α7 nAChRs in GABAergic neurons. Deletion of α7 nAChRs resulted in a reduction of radial glia-like cells within the subgranular zone of the DG and a concomitant trend towards decreased immature neurons, specifically in male mice, as well as sex-dependent changes in several behaviors, including social recognition and spatial learning. Overall, these findings suggest α7 nAChRs expressed in GABAergic neurons play an important role in regulating the adult neural stem cell pool and behavior in a sex-dependent manner. This provides important insight into the mechanisms by which cholinergic dysfunction contributes to the cognitive and behavioral changes associated with neurodevelopmental and neurodegenerative disorders.
Keywords: DCX; GFAP; Memory; Neurogenesis; Progenitor; Stem cell.
Figures

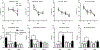
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

