Response-shift effects in neuromyelitis optica spectrum disorder: estimating response-shift-adjusted scores using equating
- PMID: 33398520
- PMCID: PMC8068715
- DOI: 10.1007/s11136-020-02727-8
Response-shift effects in neuromyelitis optica spectrum disorder: estimating response-shift-adjusted scores using equating
Abstract
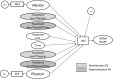
Background: In our companion paper, random intercept models (RIMs) investigated response-shift effects in a clinical trial comparing Eculizumab to Placebo for people with neuromyelitis optica spectrum disorder (NMOSD). RIMs predicted Global Health using the EQ-5D Visual Analogue Scale item (VAS) to encompass broad criteria that people might consider. The SF36™v2 mental and physical component scores (MCS and PCS) helped us detect response shift in VAS. Here, we sought to "back-translate" the VAS into the MCS/PCS scores that would have been observed if response shift had not been present.
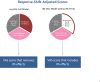
Methods: This secondary analysis utilized NMOSD clinical trial data evaluating the impact of Eculizumab in preventing relapses (n = 143). Analyses began by equating raw scores from the VAS, MCS, and PCS, and computing scores that removed response-shift effects. Correlation analysis and descriptive displays provided a more comprehensive examination of response-shift effects.
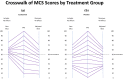
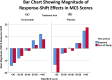
Results: MCS and PCS crosswalks with VAS equated the scores that include and exclude response-shift effects. These two sets of scores had low shared variance for MCS for both groups, suggesting that corresponding mental health constructs were substantially different. The shared variance contrast for physical health was distinct only for the Placebo group. The larger MCS response-shift effects were found at end of study for Placebo only and were more prominent at extremes of the MCS score distribution.
Conclusions: Our results reveal notable treatment group differences in MCS but not PCS response shifts, which can explain null results detected in previous work. The method introduced herein provides a way to provide further information about response-shift effects in clinical trial data.
Keywords: Clinical trial; Clinician-assessed outcome; Definitive neuromyelitis optica; Interpretation of change; Neurologic; Neuromyelitis optica spectrum disorder; Patient-reported outcome; Response shift.
Conflict of interest statement
All authors declare that they have no potential conflicts of interest and report no disclosures.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

