Nivolumab in Advanced Hepatocellular Carcinoma: Safety Profile and Select Treatment-Related Adverse Events From the CheckMate 040 Study
- PMID: 33400305
- PMCID: PMC7543234
- DOI: 10.1634/theoncologist.2019-0591
Nivolumab in Advanced Hepatocellular Carcinoma: Safety Profile and Select Treatment-Related Adverse Events From the CheckMate 040 Study
Abstract
Background: CheckMate 040 assessed the efficacy and safety of nivolumab in patients with advanced hepatocellular carcinoma (HCC). Understanding the safety profile of nivolumab is needed to support the management of treatment-related adverse events (TRAEs). This analysis assessed the safety of nivolumab monotherapy in the phase I/II, open-label CheckMate 040 study.
Materials and methods: Select TRAEs (sTRAEs; TRAEs with potential immunologic etiology requiring more frequent monitoring) occurring between first dose and 30 days after last dose were analyzed in patients in the dose-escalation and -expansion phases. Time to onset (TTO), time to resolution (TTR), and recurrence of sTRAEs were assessed, and the outcome of treatment with immune-modulating medication (IMM) was evaluated.
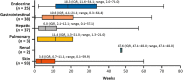
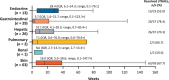
Results: The analysis included 262 patients. The most common sTRAE was skin (35.5%), followed by gastrointestinal (14.5%) and hepatic (14.1%) events; the majority were grade 1/2, with 10.7% of patients experiencing grade 3/4 events. One patient had grade 5 pneumonitis. Median (range) TTO ranged from 3.6 (0.1-59.9) weeks for skin sTRAEs to 47.6 (47.1-48.0) weeks for renal sTRAEs. Overall, 68% of sTRAEs resolved, with median (range) TTR ranging from 3.7 (0.1-123.3+) weeks for gastrointestinal sTRAEs to 28.4 (0.1-79.1) weeks for endocrine sTRAEs. Most gastrointestinal and all hepatic events resolved with treatment in accordance with established toxicity management algorithms. In 57 patients (40%), sTRAEs were managed with IMM. Reoccurrence of sTRAEs was uncommon following rechallenge with nivolumab.
Conclusion: Nivolumab demonstrated a manageable safety profile in this analysis of patients with advanced HCC. A majority of sTRAEs resolved with treatment.
Implications for practice: Nivolumab is a viable treatment option for patients with previously treated advanced hepatocellular carcinoma as it has demonstrated durable tumor responses and promising survival. Nivolumab has a manageable safety profile. The most common select treatment-related adverse events (sTRAEs) in this analysis were skin related (35%). Gastrointestinal and hepatic sTRAEs were observed in approximately 14% of patients. The majority of sTRAEs resolved (68%). Safety events are easier to manage if addressed early. Patient education on signs and symptoms to watch out for and the importance of early reporting and consultation should be emphasized.
Keywords: Adverse drug event; Hepatocellular carcinoma; Immunotherapy; Nivolumab; PD‐1 inhibitor.
© 2020 The Authors. The Oncologist published by Wiley Periodicals LLC on behalf of AlphaMed Press.
Conflict of interest statement
Figures

References
-
- Llovet JM, Zucman‐Rossi J, Pikarsky E et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. - PubMed
-
- Ferlay J, Soerjomataram I, Ervik M et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Available at https://publications.iarc.fr/Databases/Iarc‐Cancerbases/GLOBOCAN‐2012‐Es.... Accessed January 20, 2020.
-
- Bristol Myers Squibb OPDIVO (nivolumab) [package insert]. Bristol Myers Squibb, Princeton, NJ; 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

