STRA8 induces transcriptional changes in germ cells during spermatogonial development
- PMID: 33400349
- PMCID: PMC7920925
- DOI: 10.1002/mrd.23448
STRA8 induces transcriptional changes in germ cells during spermatogonial development
Abstract
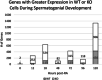
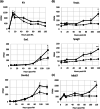
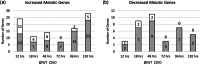
Spermatogonial development is a key process during spermatogenesis to prepare germ cells to enter meiosis. While the initial point of spermatogonial differentiation is well-characterized, the development of spermatogonia from the onset of differentiation to the point of meiotic entry has not been well defined. Further, STRA8 is highly induced at the onset of spermatogonial development but its function in spermatogonia has not been defined. To better understand how STRA8 impacts spermatogonia, we performed RNA-sequencing in both wild-type and STRA8 knockout mice at multiple timepoints during retinoic acid (RA)-stimulated spermatogonial development. As expected, in spermatogonia from wild-type mice we found that steady-state levels of many transcripts that define undifferentiated progenitor cells were decreased while transcripts that define the differentiating spermatogonia were increased as a result of the actions of RA. However, the spermatogonia from STRA8 knockout mice displayed a muted RA response such that there were more transcripts typical of undifferentiated cells and fewer transcripts typical of differentiating cells following RA action. While spermatogonia from STRA8 knockout mice can ultimately form spermatocytes that fail to complete meiosis, it appears that the defect likely begins as a result of altered messenger RNA levels during spermatogonial differentiation.
Keywords: STRA8; retinoic acid; spermatogenesis; spermatogonia; testis.
© 2021 The Authors. Molecular Reproduction and Development published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures



References
-
- Agrimson, K. S. , Onken, J. , Mitchell, D. , Topping, T. B. , Chiarini‐Garcia, H. , Hogarth, C. A. , & Griswold, M. D. (2016). Characterizing the spermatogonial response to retinoic acid during the onset of spermatogenesis and following synchronization in the neonatal mouse testis. Biology of Reproduction, 95(4), 81. 10.1095/biolreprod.116.141770 - DOI - PMC - PubMed
-
- Anderson, E. L. , Baltus, A. E. , Roepers‐Gajadien, H. L. , Hassold, T. J. , de Rooij, D. G. , van Pelt, A. M. M. , & Page, D. C. (2008). Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America, 105(39), 14976–14980. 10.1073/pnas.0807297105 - DOI - PMC - PubMed
-
- Aravin, A. A. , Sachidanandam, R. , Bourc'his, D. , Schaefer, C. , Pezic, D. , Fejes Toth, K. , Bestor, T. , & Hannon, G. J. (2008). A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Molecular Cell, 31(6), 785–799. 10.1016/j.molcel.2008.09.003 - DOI - PMC - PubMed
-
- Arnold, S. L. M. , Kent, T. , Hogarth, C. A. , Griswold, M. D. , Amory, J. K. , & Isoherranen, N. (2015). Pharmacological inhibition of ALDH1A in mice decreases all‐trans retinoic acid concentrations in a tissue specific manner. Biochemical Pharmacology, 95(3), 177–192. 10.1016/j.bcp.2015.03.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

