Psychedelic-like Properties of Quipazine and Its Structural Analogues in Mice
- PMID: 33400504
- PMCID: PMC7933111
- DOI: 10.1021/acschemneuro.0c00291
Psychedelic-like Properties of Quipazine and Its Structural Analogues in Mice
Abstract
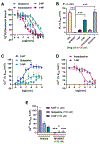
Known classic psychedelic serotonin 2A receptor (5-HT2AR) agonists retain a tryptamine or phenethylamine at their structural core. However, activation of the 5-HT2AR can be elicited by drugs lacking these fundamental scaffolds. Such is the case of the N-substituted piperazine quipazine. Here, we show that quipazine bound to and activated 5-HT2AR as measured by [3H]ketanserin binding displacement, Ca2+ mobilization, and accumulation of the canonical Gq/11 signaling pathway mediator inositol monophosphate (IP1) in vitro and in vivo. Additionally, quipazine induced via 5-HT2AR an expression pattern of immediate early genes (IEG) in the mouse somatosensory cortex consistent with that of classic psychedelics. In the mouse head-twitch response (HTR) model of psychedelic-like action, quipazine produced a lasting effect with high maximal responses during the peak effect that were successfully blocked by the 5-HT2AR antagonist M100907 and absent in 5-HT2AR knockout (KO) mice. The acute effect of quipazine on HTR appeared to be unaffected by serotonin depletion and was independent from 5-HT3R activation. Interestingly, some of these features were shared by its deaza bioisostere 2-NP, but not by other closely related piperazine congeners, suggesting that quipazine might represent a distinct cluster within the family of psychoactive piperazines. Together, our results add to the mounting evidence that quipazine's profile matches that of classic psychedelic 5-HT2AR agonists at cellular signaling and behavioral pharmacology levels.
Keywords: Psychedelics; animal models; pharmacology; piperazines; quipazine; serotonin receptors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

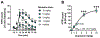


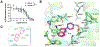



Similar articles
-
Tolerance and Cross-Tolerance among Psychedelic and Nonpsychedelic 5-HT2A Receptor Agonists in Mice.ACS Chem Neurosci. 2022 Aug 17;13(16):2436-2448. doi: 10.1021/acschemneuro.2c00170. Epub 2022 Jul 28. ACS Chem Neurosci. 2022. PMID: 35900876 Free PMC article.
-
Differences across sexes on head-twitch behavior and 5-HT2A receptor signaling in C57BL/6J mice.Neurosci Lett. 2022 Sep 25;788:136836. doi: 10.1016/j.neulet.2022.136836. Epub 2022 Aug 10. Neurosci Lett. 2022. PMID: 35963476 Free PMC article.
-
Effects of the 5-HT2A receptor antagonist volinanserin on head-twitch response and intracranial self-stimulation depression induced by different structural classes of psychedelics in rodents.Psychopharmacology (Berl). 2022 Jun;239(6):1665-1677. doi: 10.1007/s00213-022-06092-x. Epub 2022 Mar 2. Psychopharmacology (Berl). 2022. PMID: 35233648 Free PMC article.
-
Serotonin 2A Receptor (5-HT2AR) Agonists: Psychedelics and Non-Hallucinogenic Analogues as Emerging Antidepressants.Chem Rev. 2024 Jan 10;124(1):124-163. doi: 10.1021/acs.chemrev.3c00375. Epub 2023 Nov 30. Chem Rev. 2024. PMID: 38033123 Review.
-
In vitro assays for the functional characterization of (psychedelic) substances at the serotonin receptor 5-HT2A R.J Neurochem. 2022 Jul;162(1):39-59. doi: 10.1111/jnc.15570. Epub 2022 Jan 17. J Neurochem. 2022. PMID: 34978711 Review.
Cited by
-
Role of 5-HT2A, 5-HT2C, 5-HT1A and TAAR1 Receptors in the Head Twitch Response Induced by 5-Hydroxytryptophan and Psilocybin: Translational Implications.Int J Mol Sci. 2022 Nov 16;23(22):14148. doi: 10.3390/ijms232214148. Int J Mol Sci. 2022. PMID: 36430623 Free PMC article.
-
Sex-specific role for serotonin 5-HT2A receptor in modulation of opioid-induced antinociception and reward in mice.Neuropharmacology. 2022 May 15;209:108988. doi: 10.1016/j.neuropharm.2022.108988. Epub 2022 Feb 17. Neuropharmacology. 2022. PMID: 35183539 Free PMC article.
-
Mechanisms and molecular targets surrounding the potential therapeutic effects of psychedelics.Mol Psychiatry. 2023 Sep;28(9):3595-3612. doi: 10.1038/s41380-023-02274-x. Epub 2023 Sep 27. Mol Psychiatry. 2023. PMID: 37759040 Free PMC article. Review.
-
Molecular targets of psychedelic-induced plasticity.J Neurochem. 2022 Jul;162(1):80-88. doi: 10.1111/jnc.15536. Epub 2021 Nov 15. J Neurochem. 2022. PMID: 34741320 Free PMC article. Review.
-
Tolerance and Cross-Tolerance among Psychedelic and Nonpsychedelic 5-HT2A Receptor Agonists in Mice.ACS Chem Neurosci. 2022 Aug 17;13(16):2436-2448. doi: 10.1021/acschemneuro.2c00170. Epub 2022 Jul 28. ACS Chem Neurosci. 2022. PMID: 35900876 Free PMC article.
References
-
- Glennon RA (1994) Classical Hallucinogens: An Introductory Overview. NIDA Res. Monogr 176, 4–32. - PubMed
-
- Cooper JR, Smelser NJ, and Baltes PB (2001) Neurotransmitters. Int. Encycl Soc. Behav. Sci, 10612–10619.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

