Benzophenone-3 promotion of mammary tumorigenesis is diet-dependent
- PMID: 33400736
- PMCID: PMC7721615
- DOI: 10.18632/oncotarget.27831
Benzophenone-3 promotion of mammary tumorigenesis is diet-dependent
Abstract
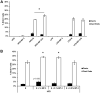
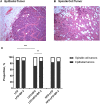
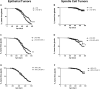
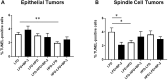
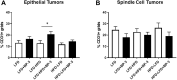
Benzophenone-3 is a putative endocrine disrupting chemical and common ingredient in sunscreens. The potential of endocrine disrupting chemicals to act as agonists or antagonists in critical hormonally regulated processes, such as mammary gland development and mammary tumorigenesis, demands evaluation of its potential in promoting breast cancer. This study identifies the effects of BP-3 on mammary tumorigenesis with high-fat diet during puberty versus adulthood in Trp53-null transplant BALB/c mice. Benzophenone-3 exposure yielded levels in urine similar to humans subjected to heavy topical sunscreen exposure. Benzophenone-3 was protective for epithelial tumorigenesis in mice fed lifelong low-fat diet, while promotional for epithelial tumorigenesis in mice fed adult high-fat diet. Benzophenone-3 increased tumor cell proliferation, decreased tumor cell apoptosis, and increased tumor vascularity dependent on specific dietary regimen and tumor histopathology. Even in instances of an ostensibly protective effect, other parameters suggest greater risk. Although benzophenone-3 seemed protective on low-fat diet, spindle cell tumors arising in these mice showed increased proliferation and decreased apoptosis. This points to a need for further studies of benzophenone-3 in both animal models and humans as a potential breast cancer risk factor, as well as a more general need to evaluate endocrine disrupting chemicals in varying dietary contexts.
Keywords: benzophenone-3; breast cancer; dietary animal fat; mammary tumorigenesis; oxybenzone.
Copyright: © 2020 Kariagina et al.
Conflict of interest statement
CONFLICTS OF INTEREST Authors have no conflicts of interest to declare.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

