Prevention of Respiratory Syncytial Virus Infection in Healthy Adults by a Single Immunization of Ad26.RSV.preF in a Human Challenge Study
- PMID: 33400792
- PMCID: PMC9417128
- DOI: 10.1093/infdis/jiab003
Prevention of Respiratory Syncytial Virus Infection in Healthy Adults by a Single Immunization of Ad26.RSV.preF in a Human Challenge Study
Abstract
Background: Respiratory syncytial virus (RSV) is a significant cause of severe lower respiratory tract disease in children and older adults, but has no approved vaccine. This study assessed the potential of Ad26.RSV.preF to protect against RSV infection and disease in an RSV human challenge model.
Methods: In this double-blind, placebo-controlled study, healthy adults aged 18-50 years were randomized 1:1 to receive 1 × 1011 vp Ad26.RSV.preF or placebo intramuscularly. Twenty-eight days postimmunization, volunteers were challenged intranasally with RSV-A (Memphis 37b). Assessments included viral load (VL), RSV infections, clinical symptom score (CSS), safety, and immunogenicity.
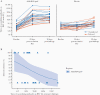
Results: Postchallenge, VL, RSV infections, and disease severity were lower in Ad26.RSV.preF (n = 27) vs placebo (n = 26) recipients: median VL area under the curve (AUC) quantitative real-time polymerase chain reaction: 0.0 vs 236.0 (P = .012; predefined primary endpoint); median VL-AUC quantitative culture: 0.0 vs 109; RSV infections 11 (40.7%) vs 17 (65.4%); median RSV AUC-CSS 35 vs 167, respectively. From baseline to 28 days postimmunization, geometric mean fold increases in RSV A2 neutralizing antibody titers of 5.8 and 0.9 were observed in Ad26.RSV.preF and placebo, respectively. Ad26.RSV.preF was well tolerated.
Conclusions: Ad26.RSV.preF demonstrated protection from RSV infection through immunization in a human challenge model, and therefore could potentially protect against natural RSV infection and disease.
Clinical trials registration: NCT03334695; CR108398, 2017-003194-33 (EudraCT); VAC18193RSV2002.
Keywords: Pre-F protein; RSV fusion protein; adenoviral vectors; adults; challenge study; respiratory syncytial virus; vaccine.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



References
-
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. - PubMed
-
- Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2020; 222:S577–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

