Utility of the antigen test for coronavirus disease 2019: Factors influencing the prediction of the possibility of disease transmission
- PMID: 33401037
- PMCID: PMC7778366
- DOI: 10.1016/j.ijid.2020.12.079
Utility of the antigen test for coronavirus disease 2019: Factors influencing the prediction of the possibility of disease transmission
Erratum in
-
Corrigendum to "Utility of the antigen test for coronavirus disease 2019: factors influencing the prediction of the possibility of disease transmission" [Int J Infect Dis 104 (2021) 65-72].Int J Infect Dis. 2021 Aug;109:323. doi: 10.1016/j.ijid.2021.06.009. Epub 2021 Jun 23. Int J Infect Dis. 2021. PMID: 34172380 Free PMC article. No abstract available.
Abstract
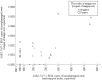
Objectives: Rapid antigen testing (RAT) for coronavirus disease 2019 (COVID-19) has lower sensitivity but high accuracy during the early stage when compared to reverse transcription quantitative polymerase chain reaction (RT-qPCR). The aim of this study was to investigate the concordance between RAT and RT-qPCR results, and their prediction of disease transmission.
Methods: This single-center retrospective observational study of inpatients with COVID-19 was conducted from March 6 to June 14, 2020. Nasopharyngeal swabs were used to perform RAT and RT-qPCR. The primary endpoint was concordance between RAT and RT-qPCR results. The secondary endpoints were the factors causing disagreement in the results and the estimated transmissibility in RT-qPCR-positive patients with mild symptoms.
Results: Overall, 229 samples in viral transport medium (VTM) were obtained from 105 patients. The positive and negative concordance rates for VTM were 41% vs 99% (κ = 0.37) and 72% vs 100% (κ = 0.50) for samples collected on disease days 2-9. An increased body temperature (odds ratio 0.54) and absence of drugs with potential antiviral effect (odds ratio 0.48) yielded conflicting results. RAT was associated with the ability to end isolation (OR 0.11, 95% confidence interval 0.20-0.61).
Conclusions: RAT and RT-qPCR results were highly consistent for samples collected at the appropriate time and could be useful for inferring the possibility of transmissibility.
Keywords: Antigen test; Appropriate timing for antigen test; COVID-19; Estimating transmissibility.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Amin M., Abbas G. Docking study of chloroquine and hydroxychloroquine interaction with RNA binding domain of nucleocapsid phospho–protein—an in silico insight into the comparative efficacy of repurposing antiviral drugs. J Biomol Struct Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1775703. preprint. - DOI - PubMed
-
- Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention . 2020. Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19. Available at: https://www.cdc.gov/coronavirus/2019–ncov/community/strategy–discontinue.... [Accessed 14 August 2020] (Updated July 22, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

