Subcutaneous Implantation Assessment of New Calcium-Silicate Based Sealer for Warm Obturation
- PMID: 33401424
- PMCID: PMC7824331
- DOI: 10.3390/biomedicines9010024
Subcutaneous Implantation Assessment of New Calcium-Silicate Based Sealer for Warm Obturation
Abstract
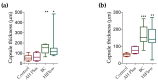
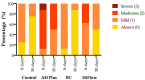
Calcium silicate-based sealers were recently introduced as a new class of endodontic sealers, with potential further benefits due to their bioactivity. The aim of this study was to evaluate the biocompatibility of two new hydraulic calcium silicate-based sealers, TotalFill BC Sealer (FKG, La Chaux-des-Fonds, Switzerland) and TotalFill BC Sealer HiFlow (FKG, La Chaux-des-Fonds, Switzerland) through subcutaneous implantation in connective tissue of rats. Subcutaneous implantation was performed in 16 young Wistar rats. Four polyethylene tubes were implanted in each animal, one empty to serve as a control, and three filled with tested sealers: AH Plus as reference (Dentsply DeTrey, Konstanz, Germany), TotalFill BC Sealer (BC) and TotalFill BC Sealer HiFlow (HiFlow). Eight rats were euthanized at 8 days and the remaining eight at 30 days. Hematoxylin-eosin staining was used to score the inflammatory reaction, macrophage infiltrate and to measure the thickness of the fibrous capsule. von Kossa staining was performed to evaluate the mineralization level. Kruskal-Wallis test followed by Dunn's post hoc test was used to analyze non-parametric data. To analyze the influence of the implantation time within each material, a Mann-Whitney U test was performed. At eight days post-implantation, AH Plus induced a more intense inflammatory reaction when compared both with the control (p ≤ 0.001) and BC (p ≤ 0.01). HiFlow presented a higher score of macrophage infiltrate than control (p ≤ 0.01) and BC (p ≤ 0.05). The fibrous capsule thickness in this period was significantly higher for the BC group when compared to control (p ≤ 0.01) and AH Plus (p ≤ 0.05). The mineralization potential was higher for the HiFlow group when compared with the control (p ≤ 0.001) and AH Plus (p ≤ 0.001). At 30 days post-implantation, the score for the inflammatory reaction remained higher for the AH Plus group when compared both to control (p ≤ 0.01) and BC (p ≤ 0.001). The macrophage infiltrate of the HiFlow was significantly higher than control (p ≤ 0.001) and AH Plus groups (p ≤ 0.01), additionally, the fibrous capsule of the BC (p ≤ 0.001) and HiFlow (p ≤ 0.01) groups were both thicker than control. Mineralization potential was observed only on BC (p ≤ 0.05) and HiFlow groups (p ≤ 0.001), when compared to control). BC exhibited the best biocompatibility performance of all tested sealers and HiFlow provided the greatest induction of mineralized tissues. Both TotalFill BC Sealer and TotalFill BC Sealer HiFlow are biocompatible and show potential bioactivity when implanted in the subcutaneous tissue. Bioactivity was not found in AH Plus.
Keywords: bioactivity potential; biocompatibility; calcium silicate-based sealers; endodontic sealers; endodontics; subcutaneous implantation.
Conflict of interest statement
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

