Human Adipose Tissue-Derived Mesenchymal Stromal Cells Inhibit CD4+ T Cell Proliferation and Induce Regulatory T Cells as Well as CD127 Expression on CD4+CD25+ T Cells
- PMID: 33401501
- PMCID: PMC7824667
- DOI: 10.3390/cells10010058
Human Adipose Tissue-Derived Mesenchymal Stromal Cells Inhibit CD4+ T Cell Proliferation and Induce Regulatory T Cells as Well as CD127 Expression on CD4+CD25+ T Cells
Abstract
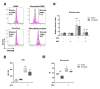
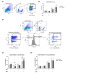
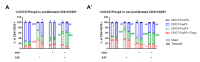
Mesenchymal stromal cells (MSC) exert their immunomodulatory potential on several cell types of the immune system, affecting and influencing the immune response. MSC efficiently inhibit T cell proliferation, reduce the secretion of pro-inflammatory cytokines, limit the differentiation of pro-inflammatory Th subtypes and promote the induction of regulatory T cells (Treg). In this study, we analyzed the immunomodulatory potential of human adipose tissue-derived MSC (ASC), on CD4+ T cells, addressing potential cell-contact dependency in relation to T cell receptor stimulation of whole human peripheral blood mononuclear cells (PBMC). ASC were cultured with not stimulated or anti-CD3/CD28-stimulated PBMC in direct and transwell cocultures; PBMC alone were used as controls. After 7 days, cocultures were harvested and we analyzed: (1) the inhibitory potential of ASC on CD4+ cell proliferation and (2) phenotypic changes in CD4+ cells in respect of Treg marker (CD25, CD127 and FoxP3) expression. We confirmed the inhibitory potential of ASC on CD4+ cell proliferation, which occurs upon PBMC stimulation and is mediated by indoleamine 2,3-dioxygenase. Importantly, ASC reduce both pro- and anti-inflammatory cytokine secretion, without indications on specific Th differentiation. We found that stimulation induces CD25 expression on CD4+ cells and that, despite inhibiting overall CD4+ cell proliferation, ASC can specifically induce the proliferation of CD4+CD25+ cells. We observed that ASC induce Treg (CD4+CD25+CD127-FoxP3+) only in not stimulated cocultures and that ASC increase the ratio of CD4+CD25+CD127+FoxP3- cells at the expense of CD4+CD25+CD127-FoxP3- cells. Our study provides new insights on the interplay between ASC and CD4+ T cells, proposing that ASC-dependent induction of Treg depends on PBMC activation which affects the balance between the different subpopulations of CD4+CD25+ cells expressing CD127 and/or FoxP3.
Keywords: CD127; CD4 T cells; immunomodulation; mesenchymal stromal cells; regulatory T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Human adipose-tissue derived mesenchymal stem cells induce functional de-novo regulatory T cells with methylated FOXP3 gene DNA.Clin Exp Immunol. 2013 Aug;173(2):343-54. doi: 10.1111/cei.12120. Clin Exp Immunol. 2013. PMID: 23607314 Free PMC article.
-
Reduced frequency and functional defects of CD4+CD25highCD127low/- regulatory T cells in patients with unexplained recurrent spontaneous abortion.Reprod Biol Endocrinol. 2020 Jun 10;18(1):62. doi: 10.1186/s12958-020-00619-7. Reprod Biol Endocrinol. 2020. PMID: 32522204 Free PMC article.
-
Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells.Stem Cell Res Ther. 2013 Jun 4;4(3):65. doi: 10.1186/scrt216. Stem Cell Res Ther. 2013. PMID: 23734780 Free PMC article.
-
Prevention of allograft rejection by amplification of Foxp3(+)CD4(+)CD25(+) regulatory T cells.Transl Res. 2009 Feb;153(2):60-70. doi: 10.1016/j.trsl.2008.12.001. Epub 2008 Dec 25. Transl Res. 2009. PMID: 19138650 Free PMC article. Review.
-
Regulatory T cell in stroke: a new paradigm for immune regulation.Clin Dev Immunol. 2013;2013:689827. doi: 10.1155/2013/689827. Epub 2013 Aug 4. Clin Dev Immunol. 2013. PMID: 23983771 Free PMC article. Review.
Cited by
-
Mesenchymal Stem Cell Induced Foxp3(+) Tregs Suppress Effector T Cells and Protect against Retinal Ischemic Injury.Cells. 2021 Nov 4;10(11):3006. doi: 10.3390/cells10113006. Cells. 2021. PMID: 34831229 Free PMC article.
-
Impact of platelet lysate on immunoregulatory characteristics of equine mesenchymal stromal cells.Front Vet Sci. 2024 Apr 24;11:1385395. doi: 10.3389/fvets.2024.1385395. eCollection 2024. Front Vet Sci. 2024. PMID: 38725585 Free PMC article.
-
Impact of Adipose-Derived Mesenchymal Stem Cells (ASCs) of Rheumatic Disease Patients on T Helper Cell Differentiation.Int J Mol Sci. 2022 May 10;23(10):5317. doi: 10.3390/ijms23105317. Int J Mol Sci. 2022. PMID: 35628127 Free PMC article.
-
Mesenchymal Stromal Cells "Think" Globally, but Act Locally.Cells. 2023 Feb 3;12(3):509. doi: 10.3390/cells12030509. Cells. 2023. PMID: 36766851 Free PMC article.
-
Adipose-derived stem cells in immune-related skin disease: a review of current research and underlying mechanisms.Stem Cell Res Ther. 2024 Feb 8;15(1):37. doi: 10.1186/s13287-023-03561-8. Stem Cell Res Ther. 2024. PMID: 38331803 Free PMC article. Review.
References
-
- Gonzalez-Rey E., Gonzalez M.A., Varela N., O’Valle F., Hernandez-Cortes P., Rico L., Büscher D., Delgado M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann. Rheum. Dis. 2010;69:241. doi: 10.1136/ard.2008.101881. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

