Durable Oral Biofilm Resistance of 3D-Printed Dental Base Polymers Containing Zwitterionic Materials
- PMID: 33401545
- PMCID: PMC7795277
- DOI: 10.3390/ijms22010417
Durable Oral Biofilm Resistance of 3D-Printed Dental Base Polymers Containing Zwitterionic Materials
Abstract
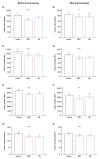
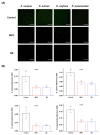
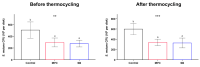
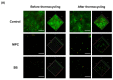
Poly(methyl methacralyate) (PMMA) has long been used in dentistry as a base polymer for dentures, and it is recently being used for the 3D printing of dental materials. Despite its many advantages, its susceptibility to microbial colonization remains to be overcome. In this study, the interface between 3D-printed PMMA specimens and oral salivary biofilm was studied following the addition of zwitterionic materials, 2-methacryloyloxyethyl phosphorylcholine (MPC) or sulfobetaine methacrylate (SB). A significant reduction in bacterial and biofilm adhesions was observed following the addition of MPC or SB, owing to their protein-repellent properties, and there were no significant differences between the two test materials. Although the mechanical properties of the tested materials were degraded, the statistical value of the reduction was minimal and all the properties fulfilled the requirements set by the International Standard, ISO 20795-2. Additionally, both the test materials maintained their resistance to biofilm when subjected to hydrothermal fatigue, with no further deterioration of the mechanical properties. Thus, novel 3D-printable PMMA incorporated with MPC or SB shows durable oral salivary biofilm resistance with maintenance of the physical and mechanical properties.
Keywords: 3D printing; dental base resin; dentistry; durability; oral salivary biofilm; poly(methyl methacralyate); zwitterion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Vojdani M., Bagheri R., Khaledi A.A.R. Effects of aluminum oxide addition on the flexural strength, surface hardness, and roughness of heat-polymerized acrylic resin. J. Dent. Sci. 2012;7:238–244. doi: 10.1016/j.jds.2012.05.008. - DOI
-
- Totu E.E., Nechifor A.C., Nechifor G., Aboul-Enein H.Y., Cristache C.M. Poly(methyl methacrylate) with TiO2 nanoparticles inclusion for stereolitographic complete denture manufacturing—The fututre in dental care for elderly edentulous patients? J. Dent. 2017;59:68–77. doi: 10.1016/j.jdent.2017.02.012. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

