Master Regulators of Muscle Atrophy: Role of Costamere Components
- PMID: 33401549
- PMCID: PMC7823551
- DOI: 10.3390/cells10010061
Master Regulators of Muscle Atrophy: Role of Costamere Components
Abstract
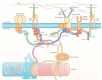
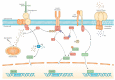
The loss of muscle mass and force characterizes muscle atrophy in several different conditions, which share the expression of atrogenes and the activation of their transcriptional regulators. However, attempts to antagonize muscle atrophy development in different experimental contexts by targeting contributors to the atrogene pathway showed partial effects in most cases. Other master regulators might independently contribute to muscle atrophy, as suggested by our recent evidence about the co-requirement of the muscle-specific chaperone protein melusin to inhibit unloading muscle atrophy development. Furthermore, melusin and other muscle mass regulators, such as nNOS, belong to costameres, the macromolecular complexes that connect sarcolemma to myofibrils and to the extracellular matrix, in correspondence with specific sarcomeric sites. Costameres sense a mechanical load and transduce it both as lateral force and biochemical signals. Recent evidence further broadens this classic view, by revealing the crucial participation of costameres in a sarcolemmal "signaling hub" integrating mechanical and humoral stimuli, where mechanical signals are coupled with insulin and/or insulin-like growth factor stimulation to regulate muscle mass. Therefore, this review aims to enucleate available evidence concerning the early involvement of costamere components and additional putative master regulators in the development of major types of muscle atrophy.
Keywords: aging; atrogene; cachexia; costamere; dystrophin; melusin; muscle atrophy; muscle disuse; nNOS; sarcopenia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pisot R., Marusic U., Biolo G., Mazzucco S., Lazzer S., Grassi B., Reggiani C., Toniolo L., di Prampero P.E., Passaro A., et al. Greater loss in muscle mass and function but smaller metabolic alterations in older compared with younger men following 2 wk of bed rest and recovery. J. Appl. Physiol. 2016;120:922–929. doi: 10.1152/japplphysiol.00858.2015. - DOI - PubMed
-
- Lang F., Khaghani S., Turk C., Wiederstein J.L., Holper S., Piller T., Nogara L., Blaauw B., Gunther S., Muller S., et al. Single Muscle Fiber Proteomics Reveals Distinct Protein Changes in Slow and Fast Fibers during Muscle Atrophy. J. Proteome Res. 2018;17:3333–3347. doi: 10.1021/acs.jproteome.8b00093. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

