Cell-Permeable Succinate Rescues Mitochondrial Respiration in Cellular Models of Statin Toxicity
- PMID: 33401621
- PMCID: PMC7796258
- DOI: 10.3390/ijms22010424
Cell-Permeable Succinate Rescues Mitochondrial Respiration in Cellular Models of Statin Toxicity
Abstract
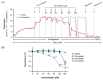
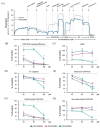
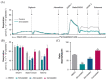
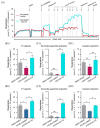
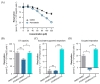
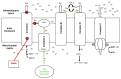
Statins are the cornerstone of lipid-lowering therapy. Although generally well tolerated, statin-associated muscle symptoms (SAMS) represent the main reason for treatment discontinuation. Mitochondrial dysfunction of complex I has been implicated in the pathophysiology of SAMS. The present study proposed to assess the concentration-dependent ex vivo effects of three statins on mitochondrial respiration in viable human platelets and to investigate whether a cell-permeable prodrug of succinate (complex II substrate) can compensate for statin-induced mitochondrial dysfunction. Mitochondrial respiration was assessed by high-resolution respirometry in human platelets, acutely exposed to statins in the presence/absence of the prodrug NV118. Statins concentration-dependently inhibited mitochondrial respiration in both intact and permeabilized cells. Further, statins caused an increase in non-ATP generating oxygen consumption (uncoupling), severely limiting the OXPHOS coupling efficiency, a measure of the ATP generating capacity. Cerivastatin (commercially withdrawn due to muscle toxicity) displayed a similar inhibitory capacity compared with the widely prescribed and tolerable atorvastatin, but did not elicit direct complex I inhibition. NV118 increased succinate-supported mitochondrial oxygen consumption in atorvastatin/cerivastatin-exposed platelets leading to normalization of coupled (ATP generating) respiration. The results acquired in isolated human platelets were validated in a limited set of experiments using atorvastatin in HepG2 cells, reinforcing the generalizability of the findings.
Keywords: HepG2 cells; NV118; cell-permeable succinate; mitochondria; platelets; statins.
Conflict of interest statement
I.C., E.Å.F., J.K.E., M.J.H. and E.E. have, or have had, salary from and/or equity interest in Abliva AB (previously named NeuroVive Pharmaceutical AB), a company active in the field of mitochondrial medicine. J.K.E., E.E., and M.J.H. have filed patent applications for the use of succinate prodrugs for treatment of lactic acidosis or drug-induced side effects due to complex I-related impairment of mitochondrial oxidative phosphorylation (WO/2015/155238) and protected carboxylic acid-based metabolites for treatment of mitochondrial disorders (WO/2017/060400, WO/2017/060418, WO/2017/060422). This does not alter our adherence to manuscript policies on sharing data and materials. Abliva AB had no role in the study design, the data collection and analysis, or the preparation of the manuscript. The remaining authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. - DOI - PubMed
-
- Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Eur. Heart J. 2019 doi: 10.1093/eurheartj/ehz455. - DOI - PubMed
-
- Ference B.A., Ginsberg H.N., Graham I., Ray K.K., Packard C.J., Bruckert E., Hegele R.A., Krauss R.M., Raal F.J., Schunkert H., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. - DOI - PMC - PubMed
-
- Stroes E.S., Thompson P.D., Corsini A., Vladutiu G.D., Raal F.J., Ray K.K., Roden M., Stein E., Tokgözoğlu L., Nordestgaard B.G., et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

