Coagulative Disorders in Critically Ill COVID-19 Patients with Acute Distress Respiratory Syndrome: A Critical Review
- PMID: 33401632
- PMCID: PMC7795033
- DOI: 10.3390/jcm10010140
Coagulative Disorders in Critically Ill COVID-19 Patients with Acute Distress Respiratory Syndrome: A Critical Review
Abstract
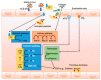
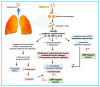
In critically ill patients with acute respiratory distress syndrome (ARDS) coronavirus disease 2019 (COVID-19), a high incidence of thromboembolic and hemorrhagic events is reported. COVID-19 may lead to impairment of the coagulation cascade, with an imbalance in platelet function and the regulatory mechanisms of coagulation and fibrinolysis. Clinical manifestations vary from a rise in laboratory markers and subclinical microthrombi to thromboembolic events, bleeding, and disseminated intravascular coagulation. After an inflammatory trigger, the mechanism for activation of the coagulation cascade in COVID-19 is the tissue factor pathway, which causes endotoxin and tumor necrosis factor-mediated production of interleukins and platelet activation. The consequent massive infiltration of activated platelets may be responsible for inflammatory infiltrates in the endothelial space, as well as thrombocytopenia. The variety of clinical presentations of the coagulopathy confronts the clinician with the difficult questions of whether and how to provide optimal supportive care. In addition to coagulation tests, advanced laboratory tests such as protein C, protein S, antithrombin, tissue factor pathway inhibitors, D-dimers, activated factor Xa, and quantification of specific coagulation factors can be useful, as can thromboelastography or thromboelastometry. Treatment should be tailored, focusing on the estimated risk of bleeding and thrombosis. The aim of this review is to explore the pathophysiology and clinical evidence of coagulation disorders in severe ARDS-related COVID-19 patients.
Keywords: COVID-19; acute distress respiratory syndrome; bleeding; coagulopathy; heparin; platelets; thrombosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. - DOI - PMC - PubMed
-
- Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

